Identification of chicken CAR homology as a cellular receptor for the emerging highly pathogenic fowl adenovirus 4 via unique binding mechanism
- PMID: 32174269
- PMCID: PMC7144210
- DOI: 10.1080/22221751.2020.1736954
Identification of chicken CAR homology as a cellular receptor for the emerging highly pathogenic fowl adenovirus 4 via unique binding mechanism
Abstract
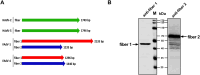
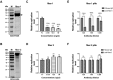
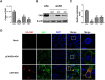
Since 2015, the prevalence of severe hepatitis-hydropericardium syndrome, which is caused by the novel genotype fowl adenovirus serotype 4 (FAdV-4), has increased in China and led to considerable economic losses. The replication cycle of FAdV-4, especially the emerging highly pathogenic novel genotype FAdV-4, remains largely unknown. The adenovirus fibre interacts with the cellular receptor as the initial step in adenovirus (AdV) infection. In our previous studies, the complete genome sequence showed that the fibre patterns of FAdV-4 were distinct from all other AdVs. Here, protein-blockage and antibody-neutralization assays were performed to confirm that the novel FAdV-4 short fibre was critical for binding to susceptible leghorn male hepatocellular (LMH) cells. Subsequently, fibre 1 was used as bait to investigate the receptor on LMH cells via mass spectrometry. The chicken coxsackie and adenovirus receptor (CAR) protein was confirmed as the novel FAdV-4 receptor in competition assays. We further identified the D2 domain of CAR (D2-CAR) as the active domain responsible for binding to the short fibre of the novel FAdV-4. Taken together, these findings demonstrate for the first time that the chicken CAR homolog is a cellular receptor for the novel FAdV-4, which facilitates viral entry by interacting with the viral short fibre through the D2 domain. Collectively, these findings provide an in-depth understanding of the mechanisms of the emerging novel genotype FAdV-4 invasion and pathogenesis.
Keywords: D2 domain; Emerging FAdV-4; cell receptor; chicken CAR; novel binding mechanism; short fibre.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
Gga-miR-30c-5p Enhances Apoptosis in Fowl Adenovirus Serotype 4-Infected Leghorn Male Hepatocellular Cells and Facilitates Viral Replication through Myeloid Cell Leukemia-1.Viruses. 2022 May 7;14(5):990. doi: 10.3390/v14050990. Viruses. 2022. PMID: 35632731 Free PMC article.
-
An Inactivated Novel Genotype Fowl Adenovirus 4 Protects Chickens against the Hydropericardium Syndrome That Recently Emerged in China.Viruses. 2017 Aug 8;9(8):216. doi: 10.3390/v9080216. Viruses. 2017. PMID: 28786949 Free PMC article.
-
Fiber-1 of serotype 4 fowl adenovirus mediates superinfection resistance against serotype 8b fowl adenovirus.Front Microbiol. 2022 Dec 21;13:1086383. doi: 10.3389/fmicb.2022.1086383. eCollection 2022. Front Microbiol. 2022. PMID: 36620032 Free PMC article.
-
Requirement of Cellular Protein CCT7 for the Replication of Fowl Adenovirus Serotype 4 (FAdV-4) in Leghorn Male Hepatocellular Cells Via Interaction with the Viral Hexon Protein.Viruses. 2019 Jan 27;11(2):107. doi: 10.3390/v11020107. Viruses. 2019. PMID: 30691230 Free PMC article.
-
Fowl adenovirus-induced diseases and strategies for their control - a review on the current global situation.Avian Pathol. 2018 Apr;47(2):111-126. doi: 10.1080/03079457.2017.1385724. Epub 2017 Oct 25. Avian Pathol. 2018. PMID: 28950714 Review.
Cited by
-
Precise location of three novel linear epitopes using the generated monoclonal antibodies against the Knob domain of FAdV-4 surface structural protein, fiber1.Front Cell Infect Microbiol. 2024 Sep 18;14:1468428. doi: 10.3389/fcimb.2024.1468428. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39359940 Free PMC article.
-
Variation in the affinity of three representative avian adenoviruses for the cellular coxsackievirus and adenovirus receptor.Vet Res. 2024 Feb 19;55(1):23. doi: 10.1186/s13567-024-01277-y. Vet Res. 2024. PMID: 38374082 Free PMC article.
-
The Role of Hexon Amino Acid 188 Varies in Fowl Adenovirus Serotype 4 Strains with Different Virulence.Microbiol Spectr. 2022 Jun 29;10(3):e0149322. doi: 10.1128/spectrum.01493-22. Epub 2022 May 19. Microbiol Spectr. 2022. PMID: 35587634 Free PMC article.
-
Hsp70 Inhibits the Replication of Fowl Adenovirus Serotype 4 by Suppressing Viral Hexon with the Assistance of DnaJC7.J Virol. 2022 Aug 10;96(15):e0080722. doi: 10.1128/jvi.00807-22. Epub 2022 Jul 19. J Virol. 2022. PMID: 35852354 Free PMC article.
-
A novel fiber-2-edited live attenuated vaccine candidate against the highly pathogenic serotype 4 fowl adenovirus.Vet Res. 2021 Feb 27;52(1):35. doi: 10.1186/s13567-021-00907-z. Vet Res. 2021. PMID: 33640033 Free PMC article.
References
-
- Benko M, Harrach B.. Molecular evolution of adenoviruses. Curr Top Microbiol Immunol. 2003;272:3–35. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
