Fictive Scratching Patterns in Brain Cortex-Ablated, Midcollicular Decerebrate, and Spinal Cats
- PMID: 32174815
- PMCID: PMC7056700
- DOI: 10.3389/fncir.2020.00001
Fictive Scratching Patterns in Brain Cortex-Ablated, Midcollicular Decerebrate, and Spinal Cats
Abstract
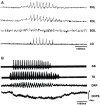
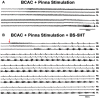
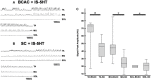
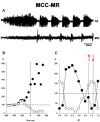
Background: The spinal cord's central pattern generators (CPGs) have been explained by the symmetrical half-center hypothesis, the bursts generator, computational models, and more recently by connectome circuits. Asymmetrical models, at odds with the half-center paradigm, are composed of extensor and flexor CPG modules. Other models include not only flexor and extensor motoneurons but also motoneuron pools controlling biarticular muscles. It is unknown whether a preferred model can explain some particularities that fictive scratching (FS) in the cat presents. The first aim of this study was to investigate FS patterns considering the aiming and the rhythmic periods, and second, to examine the effects of serotonin (5HT) on and segmental inputs to FS. Methods: The experiments were carried out first in brain cortex-ablated cats (BCAC), then spinalized (SC), and for the midcollicular (MCC) preparation. Subjects were immobilized and the peripheral nerves were used to elicit the Monosynaptic reflex (MR), to modify the scratching patterns and for electroneurogram recordings. Results: In BCAC, FS was produced by pinna stimulation and, in some cases, by serotonin. The scratching aiming phase (AP) initiates with the activation of either flexor or extensor motoneurons. Serotonin application during the AP produced simultaneous extensor and flexor bursts. Furthermore, WAY 100635 (5HT1A antagonist) produced a brief burst in the tibialis anterior (TA) nerve, followed by a reduction in its electroneurogram (ENG), while the soleus ENG remained silent. In SC, rhythmic phase (RP) activity was recorded in the soleus motoneurons. Serotonin or WAY produced FS bouts. The electrical stimulation of Ia afferent fibers produced heteronymous MRes waxing and waning during the scratch cycle. In MCC, FS began with flexor activity. Electrical stimulation of either deep peroneus (DP) or superficial peroneus (SP) nerves increased the duration of the TA electroneurogram. Medial gastrocnemius (MG) stretching or MG nerve electrical stimulation produced a reduction in the TA electroneurogram and an initial MG extensor burst. MRes waxed and waned during the scratch cycle. Conclusion: Descending pathways and segmental afferent fibers, as well as 5-HT and WAY, can change the FS pattern. To our understanding, the half-center hypothesis is the most suitable for explaining the AP in MCC.
Keywords: decerebrate cat; fictive scratching; monosynaptic reflex; motor patterns; spinal cat.
Copyright © 2020 Aguilar Garcia, Dueñas-Jiménez, Castillo, Osuna-Carrasco, De La Torre Valdovinos, Castañeda-Arellano, López-Ruiz, Toro-Castillo, Treviño, Mendizabal-Ruiz and Duenas-Jimenez.
Figures








Similar articles
-
Hind limb motoneurons activity during fictive locomotion or scratching induced by pinna stimulation, serotonin, or glutamic acid in brain cortex-ablated cats.Physiol Rep. 2017 Sep;5(18):e13458. doi: 10.14814/phy2.13458. Epub 2017 Sep 27. Physiol Rep. 2017. PMID: 28963128 Free PMC article.
-
Modulation of oligosynaptic cutaneous and muscle afferent reflex pathways during fictive locomotion and scratching in the cat.J Neurophysiol. 1998 Jan;79(1):447-63. doi: 10.1152/jn.1998.79.1.447. J Neurophysiol. 1998. PMID: 9425213
-
Control of transmission in muscle group IA afferents during fictive locomotion in the cat.J Neurophysiol. 1996 Dec;76(6):4104-12. doi: 10.1152/jn.1996.76.6.4104. J Neurophysiol. 1996. PMID: 8985904
-
Chapter 2--the spinal generation of phases and cycle duration.Prog Brain Res. 2011;188:15-29. doi: 10.1016/B978-0-444-53825-3.00007-3. Prog Brain Res. 2011. PMID: 21333800 Review.
-
Contributions to the understanding of gait control.Dan Med J. 2014 Apr;61(4):B4823. Dan Med J. 2014. PMID: 24814597 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

