Regulation of Thermogenic Adipocyte Differentiation and Adaptive Thermogenesis Through Histone Acetylation
- PMID: 32174890
- PMCID: PMC7057231
- DOI: 10.3389/fendo.2020.00095
Regulation of Thermogenic Adipocyte Differentiation and Adaptive Thermogenesis Through Histone Acetylation
Abstract
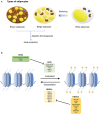
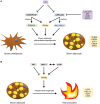
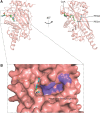
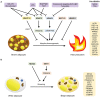
Over the past decade, the increasing prevalence of obesity and its associated metabolic disorders constitutes one of the most concerning healthcare issues for countries worldwide. In an effort to curb the increased mortality and morbidity derived from the obesity epidemic, various therapeutic strategies have been developed by researchers. In the recent years, advances in the field of adipocyte biology have revealed that the thermogenic adipose tissue holds great potential in ameliorating metabolic disorders. Additionally, epigenetic research has shed light on the effects of histone acetylation on adipogenesis and thermogenesis, thereby establishing the essential roles which histone acetyltransferases (HATs) and histone deacetylases (HDACs) play in metabolism and systemic energy homeostasis. In regard to the therapeutic potential of thermogenic adipocytes for the treatment of metabolic diseases, herein, we describe the current state of knowledge of the regulation of thermogenic adipocyte differentiation and adaptive thermogenesis through histone acetylation. Furthermore, we highlight how different HATs and HDACs maintain the epigenetic transcriptional network to mediate the pathogenesis of various metabolic comorbidities. Finally, we provide insights into recent advances of the potential therapeutic applications and development of HAT and HDAC inhibitors to alleviate these pathological conditions.
Keywords: adaptive thermogenesis; epigenetics; gene expression; histone acetylation; histone acetyltransferase inhibitors; histone deacetylase inhibitors; histone deacetylation; thermogenic adipocyte differentiation.
Copyright © 2020 Ong, Brunmeir, Zhang, Peng, Idris, Liu and Xu.
Figures



References
-
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief.(2017) 1–8. Available online at: https://www.cdc.gov/nchs/data/databriefs/db288.pdf - PubMed
-
- World Health Organization Global patterns of health risk. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. Geneva: World Health Organization; (2009). p. 9–12.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

