Clinical Relevance and Molecular Pathogenesis of the Emerging Serotypes 22F and 33F of Streptococcus pneumoniae in Spain
- PMID: 32174903
- PMCID: PMC7056674
- DOI: 10.3389/fmicb.2020.00309
Clinical Relevance and Molecular Pathogenesis of the Emerging Serotypes 22F and 33F of Streptococcus pneumoniae in Spain
Abstract
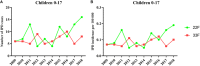
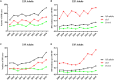
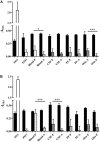
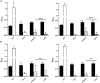
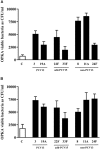
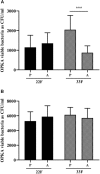
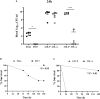
Streptococcus pneumoniae is the main bacterial cause of respiratory infections in children and the elderly worldwide. Serotype replacement is a frequent phenomenon after the introduction of conjugated vaccines, with emerging serotypes 22F and 33F as frequent non-PCV13 serotypes in children and adults in North America and other countries. Characterization of mechanisms involved in evasion of the host immune response by these serotypes is of great importance in public health because they are included in the future conjugated vaccines PCV15 and PCV20. One of the main strategies of S. pneumoniae to persistently colonize and causes infection is biofilm formation. In this study, we have evaluated the influence of capsule polysaccharide in biofilm formation and immune evasion by using clinical isolates from different sources and isogenic strains with capsules from prevalent serotypes. Since the introduction of PCV13 in Spain in the year 2010, isolates of serotypes 22F and 33F are rising among risk populations. The predominant circulating genotypes are ST43322F and ST71733F , being CC433 in 22F and CC717 in 33F the main clonal complexes in Spain. The use of clinical isolates of different origin, demonstrated that pediatric isolates of serotypes 22F and 33F formed better biofilms than adult isolates and this was statistically significant. This phenotype was greater in clinical isolates from blood origin compared to those from cerebrospinal fluid, pleural fluid and otitis. Opsonophagocytosis assays showed that serotype 22F and 33F were recognized by the PSGL-1 receptor on leukocytes, although serotype 22F, was more resistant than serotype 33F to phagocytosis killing and more lethal in a mouse sepsis model. Overall, the emergence of additional PCV15 serotypes, especially 22F, could be associated to an enhanced ability to divert the host immune response that markedly increased in a biofilm state. Our findings demonstrate that pediatric isolates of 22F and 33F, that form better biofilm than isolates from adults, could have an advantage to colonize the nasopharynx of children and therefore, be important in carriage and subsequent dissemination to the elderly. The increased ability of serotype 22F to avoid the host immune response, might explain the emergence of this serotype in the last years.
Keywords: PCV-pneumococcal conjugate vaccine; PSGL-1; Streptococcus pneumoniae; biofilms; serotype 22F; serotype 33F.
Copyright © 2020 Sempere, de Miguel, González-Camacho, Yuste and Domenech.
Figures

References
-
- Aguinagalde L., Corsini B., Domenech A., Domenech M., Camara J., Ardanuy C., et al. (2015). Emergence of amoxicillin-resistant variants of Spain9V-ST156 pneumococci expressing serotype 11A correlates with their ability to evade the host immune response. PLoS One 10:e0137565. 10.1371/journal.pone.0137565 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

