Identification of Pepper CaSBP08 Gene in Defense Response Against Phytophthora capsici Infection
- PMID: 32174944
- PMCID: PMC7054287
- DOI: 10.3389/fpls.2020.00183
Identification of Pepper CaSBP08 Gene in Defense Response Against Phytophthora capsici Infection
Abstract
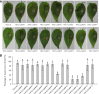
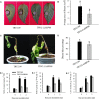
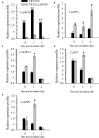
Little information is available on the role of Squamosa promoter binding protein (SBP)-box genes in pepper plants. This family of genes is known to have transcription characteristics specific to plants and to regulate plant growth, development, stress responses, and signal transduction. To investigate their specific effects in pepper (Capsicum annuum), we screened pepper SBP-box family genes (CaSBP genes) for Phytophthora capsici (P. capsici) resistance genes using virus-induced gene silencing. CaSBP08, CaSBP11, CaSBP12, and CaSBP13, which are associated with plant defense responses against P. capsici, were obtained from among fifteen identified CaSBP genes. The function of CaSBP08 was identified in pepper defense response against P. capsici infection in particular. CaSBP08 protein was localized to the nucleus. Silencing of CaSBP08 enhanced resistance to P. capsici infection. Following P. capsici inoculation, the malondialdehyde content, peroxidase activity, and disease index percentage of the CaSBP08-silenced plants decreased compared to the control. Additionally, the expression levels of other defense-related genes, especially those of CaBPR1 and CaSAR8.2, were more strongly induced in CaSBP08-silenced plants than in the control. However, CaSBP08 overexpression in Nicotiana benthamiana enhanced susceptibility to P. capsici infection. This work provides a foundation for the further research on the role of CaSBP genes in plant defense responses against P. capsici infection.
Keywords: CaSBP08; Nicotiana benthamiana; Phytophthora capsici; SBP-box family genes; defense genes; pepper.
Copyright © 2020 Zhang, Feng, Ali, Jin, Wei, Khattak and Gong.
Figures



References
LinkOut - more resources
Full Text Sources

