Sticking to the Problem: Engineering Adhesion in Molecular Endoscopic Imaging
- PMID: 32175025
- PMCID: PMC7048886
- DOI: 10.1007/s12195-020-00609-0
Sticking to the Problem: Engineering Adhesion in Molecular Endoscopic Imaging
Abstract
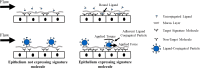
Cancers of the digestive tract cause nearly one quarter of the cancer deaths worldwide, and nearly half of these are due to cancers of the esophagus and colon. Early detection of cancer significantly increases the rate of survival, and thus it is critical that cancer within these organs is detected early. In this regard, endoscopy is routinely used to screen for transforming/cancerous (i.e. dysplastic to fully cancerous) tissue. Numerous studies have revealed that the biochemistry of the luminal surface of such tissue within the colon and esophagus becomes altered throughout disease progression. Molecular endoscopic imaging (MEI), an emerging technology, seeks to exploit these changes for the early detection of cancer. The general approach for MEI is as follows: the luminal surface of an organ is exposed to molecular ligands, or particulate probes bearing a ligand, cognate to biochemistry unique to pre-cancerous/cancerous tissue. After a wash, the tissue is imaged to determine the presence of the probes. Detection of the probes post-washing suggests pathologic tissue. In the current review we provide a succinct, but extensive, review of ligands and target moieties that could be, or are currently being investigated, as possible cognate chemistries for MEI. This is followed by a review of the biophysics that determines, in large part, the success of a particular MEI design. The work draws an analogy between MEI and the well-advanced field of cell adhesion and provides a road map for engineering MEI to achieve assays that yield highly selective recognition of transforming/cancerous tissue in situ.
Keywords: Cancer; Cell adhesion; Colon; Dysplasia; Endoscopy; Esophagus; Gastrointestinal.
© Biomedical Engineering Society 2020.
Figures


Similar articles
-
An adhesion based approach for the detection of esophageal cancer.Integr Biol (Camb). 2018 Dec 19;10(12):747-757. doi: 10.1039/c8ib00132d. Integr Biol (Camb). 2018. PMID: 30398503 Free PMC article.
-
Chromoendoscopy and magnification endoscopy for diagnosing esophageal cancer and dysplasia.Thorac Surg Clin. 2004 Feb;14(1):87-94. doi: 10.1016/S1547-4127(04)00042-8. Thorac Surg Clin. 2004. PMID: 15382312 Review.
-
AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States.Clin Gastroenterol Hepatol. 2019 Jan;17(1):16-25.e1. doi: 10.1016/j.cgh.2018.07.041. Epub 2018 Aug 2. Clin Gastroenterol Hepatol. 2019. PMID: 30077787 Review.
-
Molecular endoscopic imaging for the detection of Barrett's metaplasia using biodegradable inorganic nanoparticles: An ex-vivo pilot study on human tissue.PLoS One. 2020 Oct 1;15(10):e0239814. doi: 10.1371/journal.pone.0239814. eCollection 2020. PLoS One. 2020. PMID: 33002048 Free PMC article.
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
References
-
- Ahmed S, Strand S, Weinmann-Menke J, Urbansky L, Galle PR, Neumann H. Molecular endoscopic imaging in cancer. Dig. Endosc. 2018;30:719–729. - PubMed
-
- Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995;374:539–542. - PubMed
-
- Bird-Lieberman EL, et al. Molecular imaging using fluorescent lectins permits rapid endoscopic identification of dysplasia in Barrett’s esophagus. Nat. Med. 2012;18:315–321. - PubMed
-
- Bird-Lieberman EL, et al. Population-based study reveals new risk-stratification biomarker panel for Barrett’s esophagus. Gastroenterology. 2012;143:927–935.e3. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

