Ex-vivo lung perfusion versus standard protocol lung transplantation-mid-term survival and meta-analysis
- PMID: 32175234
- PMCID: PMC7049550
- DOI: 10.21037/acs.2020.01.02
Ex-vivo lung perfusion versus standard protocol lung transplantation-mid-term survival and meta-analysis
Abstract
Background: While extended criteria lung donation has helped expand the lung donor pool, utilization of lungs from donors of at least one other solid organ is still limited to around 15-30%. Ex-vivo lung perfusion (EVLP) offers the ability to expand the number of useable lung grafts through assessment and reconditioning of explanted lungs, particularly those not initially meeting criteria for transplantation. This meta-analysis aimed to examine the mid- to long-term survival and other short-term outcomes of patients transplanted with EVLP-treated lungs versus standard/cold-storage protocol lungs.
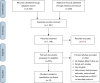
Methods: Literature search of ten medical databases was conducted for original studies involving "ex-vivo lung perfusion" and "EVLP". Included articles were assessed by two independent researchers, survival data from Kaplan-Meier curves digitized, and individual patient data imputed to conduct aggregated survival analysis. Meta-analyses of suitably reported outcomes were conducted using a random-effects model.
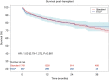
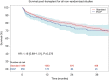
Results: Thirteen studies met inclusion criteria, with a total of 407 EVLP lung transplants and 1,765 as per standard/cold storage protocol. One study was a randomized controlled trial while the remainder were single-institution cohort series of varying design. The majority of donor lungs were from brain death donors, with EVLP lungs having significantly worse PaO2/FiO2 ratio and significantly greater rate of abnormal chest X-ray. Aggregated survival analysis of all included studies revealed no significant survival difference for EVLP or standard protocol lungs (hazard ratio 1.00; 95% confidence interval: 0.79-1.27, P=0.981). Survival at 12, 24, and 36 months for the EVLP cohort was 84%, 79%, and 74%, respectively. Survival at 12, 24, and 36 months for the standard protocol cohort was 85%, 79%, and 73%, respectively. Meta-analysis did not find a significant difference in risk of 30-day mortality or primary graft dysfunction grade 3 at 72 hours between cohorts.
Conclusions: There was no significant difference in mid- to long-term survival of EVLP lung transplant patients when compared to standard protocol donor lungs. The incidence of 30-day mortality and primary graft dysfunction grade 3 at 72 hours did not differ significantly between groups. EVLP offers the potential to increase lung donor utilization while providing similar short-term outcomes and mid- to long-term survival.
Keywords: Ex-vivo lung perfusion (EVLP); lung transplantation; meta-analysis; survival.
2020 Annals of Cardiothoracic Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous
