Corrosion Resistance and Biocompatibility Assessment of a Biodegradable Hydrothermal-Coated Mg-Zn-Ca Alloy: An in Vitro and in Vivo Study
- PMID: 32175501
- PMCID: PMC7066561
- DOI: 10.1021/acsomega.9b03889
Corrosion Resistance and Biocompatibility Assessment of a Biodegradable Hydrothermal-Coated Mg-Zn-Ca Alloy: An in Vitro and in Vivo Study
Abstract
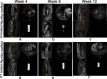
A hydrothermal (HT) coating was applied to the biomedical Mg-Zn-Ca alloy surface by microarc oxidation (MAO) and heat treatment. Then, the corrosion resistance and biocompatibility of the coated alloy was evaluated in vitro and in vivo. The corrosion rate (CR) of HT-coated implants was significantly lower in experiment. In addition, this CR increased over time in vivo but was stable, albeit higher, in vitro. The proliferation, adhesion, and live activity of bone marrow stem cells (BMSCs) were significantly greater on the surface of the HT-coated Mg alloy in vitro. Serum Mg2+ was always within the normal range in rabbits with implants, although Ca2+ was higher than normal for both uncoated and coated scaffolds. There were no significant pathological effects on the main organs of alloy-implanted rabbits compared with healthy animals. Thus, the HT coating significantly improved the corrosion resistance and biocompatibility of the Mg-Zn-Ca alloy.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


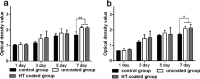






Similar articles
-
[In vivo study of a novel micro-arc oxidation coated magnesium-zinc-calcium alloy scaffold/autologous bone particles repairing critical size bone defect in rabbit].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018 Mar 15;32(3):298-305. doi: 10.7507/1002-1892.201710003. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018. PMID: 29806278 Free PMC article. Chinese.
-
Corrosion resistance and surface biocompatibility of a microarc oxidation coating on a Mg-Ca alloy.Acta Biomater. 2011 Apr;7(4):1880-9. doi: 10.1016/j.actbio.2010.11.034. Epub 2010 Dec 8. Acta Biomater. 2011. PMID: 21145440
-
Silk fibroin film-coated MgZnCa alloy with enhanced in vitro and in vivo performance prepared using surface activation.Acta Biomater. 2019 Jun;91:99-111. doi: 10.1016/j.actbio.2019.04.048. Epub 2019 Apr 24. Acta Biomater. 2019. PMID: 31028907
-
A Systematic Review and Network Meta-Analysis of Biomedical Mg Alloy and Surface Coatings in Orthopedic Application.Bioinorg Chem Appl. 2022 Mar 31;2022:4529520. doi: 10.1155/2022/4529520. eCollection 2022. Bioinorg Chem Appl. 2022. PMID: 35399618 Free PMC article. Review.
-
Biodegradable Mg-Zn-Ca-Based Metallic Glasses.Materials (Basel). 2022 Mar 15;15(6):2172. doi: 10.3390/ma15062172. Materials (Basel). 2022. PMID: 35329624 Free PMC article. Review.
Cited by
-
A Review of the Development of Titanium-Based and Magnesium-Based Metallic Glasses in the Field of Biomedical Materials.Materials (Basel). 2024 Sep 19;17(18):4587. doi: 10.3390/ma17184587. Materials (Basel). 2024. PMID: 39336328 Free PMC article. Review.
-
Insight Into Corrosion of Dental Implants: From Biochemical Mechanisms to Designing Corrosion-Resistant Materials.Curr Oral Health Rep. 2022;9(2):7-21. doi: 10.1007/s40496-022-00306-z. Epub 2022 Jan 29. Curr Oral Health Rep. 2022. PMID: 35127334 Free PMC article. Review.
-
The effect of different coatings on bone response and degradation behavior of porous magnesium-strontium devices in segmental defect regeneration.Bioact Mater. 2020 Dec 2;6(6):1765-1776. doi: 10.1016/j.bioactmat.2020.11.026. eCollection 2021 Jun. Bioact Mater. 2020. PMID: 33313453 Free PMC article.
-
Microstructure and Corrosion Characterization of a MgO/Hydroxyapatite Bilayer Coating by Plasma Electrolytic Oxidation Coupled with Flame Spraying on a Mg Alloy.ACS Omega. 2020 Sep 18;5(38):24186-24194. doi: 10.1021/acsomega.0c01574. eCollection 2020 Sep 29. ACS Omega. 2020. PMID: 33015434 Free PMC article.
-
Sol-gel synthesis of magnesium oxide nanoparticles and their evaluation as a therapeutic agent for the treatment of osteoarthritis.Nanomedicine (Lond). 2024;19(23):1867-1878. doi: 10.1080/17435889.2024.2382421. Epub 2024 Aug 7. Nanomedicine (Lond). 2024. PMID: 39109508 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
