Satellite cells and their regulation in livestock
- PMID: 32175577
- PMCID: PMC7193651
- DOI: 10.1093/jas/skaa081
Satellite cells and their regulation in livestock
Abstract
Satellite cells are the myogenic stem and progenitor population found in skeletal muscle. These cells typically reside in a quiescent state until called upon to support repair, regeneration, or muscle growth. The activities of satellite cells are orchestrated by systemic hormones, autocrine and paracrine growth factors, and the composition of the basal lamina of the muscle fiber. Several key intracellular signaling events are initiated in response to changes in the local environment causing exit from quiescence, proliferation, and differentiation. Signals emanating from Notch, wingless-type mouse mammary tumor virus integration site family members, and transforming growth factor-β proteins mediate the reversible exit from growth 0 phase while those initiated by members of the fibroblast growth factor and insulin-like growth factor families direct proliferation and differentiation. Many of these pathways impinge upon the myogenic regulatory factors (MRF), myogenic factor 5, myogenic differentiation factor D, myogenin and MRF4, and the lineage determinate, Paired box 7, to alter transcription and subsequent satellite cell decisions. In the recent past, insight into mouse transgenic models has led to a firm understanding of regulatory events that control satellite cell metabolism and myogenesis. Many of these niche-regulated functions offer subtle differences from their counterparts in livestock pointing to the existence of species-specific controls. The purpose of this review is to examine the mechanisms that mediate large animal satellite cell activity and their relationship to those present in rodents.
Keywords: livestock; myogenesis; paired box 7; satellite cell; skeletal muscle.
© The Author(s) 2020. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

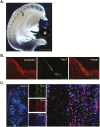
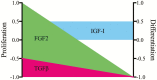
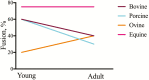
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

