Topical benzoyl peroxide for acne
- PMID: 32175593
- PMCID: PMC7077870
- DOI: 10.1002/14651858.CD011154.pub2
Topical benzoyl peroxide for acne
Abstract
Background: Acne is a common, economically burdensome condition that can cause psychological harm and, potentially, scarring. Topical benzoyl peroxide (BPO) is a widely used acne treatment; however, its efficacy and safety have not been clearly evaluated.
Objectives: To assess the effects of BPO for acne.
Search methods: We searched the following databases up to February 2019: the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We also searched five trials registers and checked the reference lists of relevant randomised controlled trials (RCTs) and systematic reviews.
Selection criteria: We included RCTs that compared topical BPO used alone (including different formulations and concentrations of BPO) or as part of combination treatment against placebo, no treatment, or other active topical medications for clinically diagnosed acne (used alone or in combination with other topical drugs not containing BPO) on the face or trunk.
Data collection and analysis: We used standard methodological procedures as expected by Cochrane. Primary outcome measures were 'participant global self-assessment of acne improvement' and 'withdrawal due to adverse events in the whole course of a trial'. 'Percentage of participants experiencing any adverse event in the whole course of a trial' was a key secondary outcome.
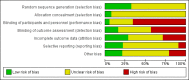
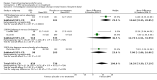
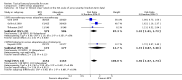
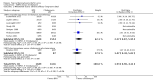
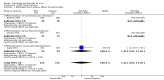
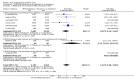
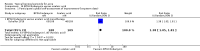
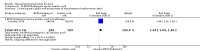
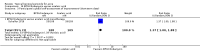
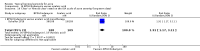
Main results: We included 120 trials (29,592 participants randomised in 116 trials; in four trials the number of randomised participants was unclear). Ninety-one studies included males and females. When reported, 72 trials included participants with mild to moderate acne, 26 included participants with severe acne, and the mean age of participants ranged from 18 to 30 years. Our included trials assessed BPO as monotherapy, as add-on treatment, or combined with other active treatments, as well as BPO of different concentrations and BPO delivered through different vehicles. Comparators included different concentrations or formulations of BPO, placebo, no treatment, or other active treatments given alone or combined. Treatment duration in 80 trials was longer than eight weeks and was only up to 12 weeks in 108 trials. Industry funded 50 trials; 63 trials did not report funding. We commonly found high or unclear risk of performance, detection, or attrition bias. Trial setting was under-reported but included hospitals, medical centres/departments, clinics, general practices, and student health centres. We reported on outcomes assessed at the end of treatment, and we classified treatment periods as short-term (two to four weeks), medium-term (five to eight weeks), or long-term (longer than eight weeks). For 'participant-reported acne improvement', BPO may be more effective than placebo or no treatment (risk ratio (RR) 1.27, 95% confidence interval (CI) 1.12 to 1.45; 3 RCTs; 2234 participants; treatment for 10 to 12 weeks; low-certainty evidence). Based on low-certainty evidence, there may be little to no difference between BPO and adapalene (RR 0.99, 95% CI 0.90 to 1.10; 5 RCTs; 1472 participants; treatment for 11 to 12 weeks) or between BPO and clindamycin (RR 0.95, 95% CI 0.68 to 1.34; 1 RCT; 240 participants; treatment for 10 weeks) (outcome not reported for BPO versus erythromycin or salicylic acid). For 'withdrawal due to adverse effects', risk of treatment discontinuation may be higher with BPO compared with placebo or no treatment (RR 2.13, 95% CI 1.55 to 2.93; 24 RCTs; 13,744 participants; treatment for 10 to 12 weeks; low-certainty evidence); the most common causes of withdrawal were erythema, pruritus, and skin burning. Only very low-certainty evidence was available for the following comparisons: BPO versus adapalene (RR 1.85, 95% CI 0.94 to 3.64; 11 RCTs; 3295 participants; treatment for 11 to 24 weeks; causes of withdrawal not clear), BPO versus clindamycin (RR 1.93, 95% CI 0.90 to 4.11; 8 RCTs; 3330 participants; treatment for 10 to 12 weeks; causes of withdrawal included local hypersensitivity, pruritus, erythema, face oedema, rash, and skin burning), erythromycin (RR 1.00, 95% CI 0.07 to 15.26; 1 RCT; 60 participants; treatment for 8 weeks; withdrawal due to dermatitis), and salicylic acid (no participants had adverse event-related withdrawal; 1 RCT; 59 participants; treatment for 12 weeks). There may be little to no difference between these groups in terms of withdrawal; however, we are unsure of the results because the evidence is of very low certainty. For 'proportion of participants experiencing any adverse event', very low-certainty evidence leaves us uncertain about whether BPO increased adverse events when compared with placebo or no treatment (RR 1.40, 95% CI 1.15 to 1.70; 21 RCTs; 11,028 participants; treatment for 10 to 12 weeks), with adapalene (RR 0.71, 95% CI 0.50 to 1.00; 7 RCTs; 2120 participants; treatment for 11 to 24 weeks), with erythromycin (no participants reported any adverse events; 1 RCT; 89 participants; treatment for 10 weeks), or with salicylic acid (RR 4.77, 95% CI 0.24 to 93.67; 1 RCT; 41 participants; treatment for 6 weeks). Moderate-certainty evidence shows that the risk of adverse events may be increased for BPO versus clindamycin (RR 1.24, 95% CI 0.97 to 1.58; 6 RCTs; 3018 participants; treatment for 10 to 12 weeks); however, the 95% CI indicates that BPO might make little to no difference. Most reported adverse events were mild to moderate, and local dryness, irritation, dermatitis, erythema, application site pain, and pruritus were the most common.
Authors' conclusions: Current evidence suggests that BPO as monotherapy or add-on treatment may be more effective than placebo or no treatment for improving acne, and there may be little to no difference between BPO and either adapalene or clindamycin. Our key efficacy evidence is based on participant self-assessment; trials of BPO versus erythromycin or salicylic acid did not report this outcome. For adverse effects, the evidence is very uncertain regarding BPO compared with adapalene, erythromycin, or salicylic acid. However, risk of treatment discontinuation may be higher with BPO compared with placebo or no treatment. Withdrawal may be linked to tolerability rather than to safety. Risk of mild to moderate adverse events may be higher with BPO compared with clindamycin. Further trials should assess the comparative effects of different preparations or concentrations of BPO and combination BPO versus monotherapy. These trials should fully assess and report adverse effects and patient-reported outcomes measured on a standardised scale.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Zhirong Yang: nothing to declare.
Yuan Zhang: nothing to declare.
Elvira Lazic Mosler: nothing to declare.
Jing Hu: nothing to declare.
Hang Li: nothing to declare.
Yanchang Zhang: nothing to declare.
Jia Liu: nothing to declare.
Qian Zhang: nothing to declare.
Figures






























































































































































Update of
- doi: 10.1002/14651858.CD011154
Similar articles
-
Topical azelaic acid, salicylic acid, nicotinamide, sulphur, zinc and fruit acid (alpha-hydroxy acid) for acne.Cochrane Database Syst Rev. 2020 May 1;5(5):CD011368. doi: 10.1002/14651858.CD011368.pub2. Cochrane Database Syst Rev. 2020. PMID: 32356369 Free PMC article.
-
Oral isotretinoin for acne.Cochrane Database Syst Rev. 2018 Nov 24;11(11):CD009435. doi: 10.1002/14651858.CD009435.pub2. Cochrane Database Syst Rev. 2018. PMID: 30484286 Free PMC article.
-
Interventions for bacterial folliculitis and boils (furuncles and carbuncles).Cochrane Database Syst Rev. 2021 Feb 26;2(2):CD013099. doi: 10.1002/14651858.CD013099.pub2. Cochrane Database Syst Rev. 2021. PMID: 33634465 Free PMC article.
-
Interventions for pityriasis rosea.Cochrane Database Syst Rev. 2019 Oct 30;2019(10):CD005068. doi: 10.1002/14651858.CD005068.pub3. Cochrane Database Syst Rev. 2019. PMID: 31684696 Free PMC article.
-
Interventions for chronic palmoplantar pustulosis.Cochrane Database Syst Rev. 2020 Jan 20;1(1):CD011628. doi: 10.1002/14651858.CD011628.pub2. Cochrane Database Syst Rev. 2020. PMID: 31958161 Free PMC article.
Cited by
-
Efficacy of antimicrobial washes before shoulder surgery against Cutibacterium: a systematic review and meta-analysis.JSES Rev Rep Tech. 2022 Mar 17;2(3):315-322. doi: 10.1016/j.xrrt.2022.02.002. eCollection 2022 Aug. JSES Rev Rep Tech. 2022. PMID: 37588870 Free PMC article. Review.
-
Unveiling the Nuances of Adult Female Acne: A Comprehensive Exploration of Epidemiology, Treatment Modalities, Dermocosmetics, and the Menopausal Influence.Int J Womens Health. 2024 Apr 18;16:663-678. doi: 10.2147/IJWH.S431523. eCollection 2024. Int J Womens Health. 2024. PMID: 38650835 Free PMC article. Review.
-
Impact of Topical Vehicles and Cutaneous Delivery Technologies on Patient Adherence and Treatment Outcomes in Acne and Rosacea.J Clin Aesthet Dermatol. 2023 May;16(5):26-34. J Clin Aesthet Dermatol. 2023. PMID: 37288283 Free PMC article. Review.
-
Effectiveness of Standard Therapy for Acne Vulgaris Based on Clinical Practice Guidelines in Indonesia.Clin Cosmet Investig Dermatol. 2024 Sep 28;17:2165-2175. doi: 10.2147/CCID.S469143. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 39364260 Free PMC article.
-
Prospective Evaluation of a Topical Botanical Skin Care Regimen on Mild to Moderate Facial and Truncal Acne and Mood.J Clin Med. 2023 Feb 13;12(4):1484. doi: 10.3390/jcm12041484. J Clin Med. 2023. PMID: 36836020 Free PMC article.
References
References to studies included in this review
Babaeinejad 2013 {published data only}
-
- ACTRN12612001006831. A randomized, double‐blind, clinical trial to compare the efficacy (lesion number), side effects, and patient's satisfaction rate of topical adapalene and benzoyl peroxide in the cases with mild acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12612001006831 (first received 18 September 2012).
-
- Babaeinejad SH, Fouladi RF. The efficacy, safety and tolerability of adapalene versus benzoyl peroxide in the treatment of mild acne vulgaris; a randomized trial. Journal of Drugs in Dermatology 2013;12(9):1033‐8. [CENTRAL: CN‐00917924; MEDLINE: ] - PubMed
Bassett 1990 {published data only}
-
- Bassett IB, Pannowitz DL, Barnetson RS. A comparative study of tea‐tree oil versus benzoyl peroxide in the treatment of acne. Medical Journal of Australia 1990;153(8):455‐8. [CENTRAL: CN‐00070530; MEDLINE: ] - PubMed
Bikowski 2006 {published data only}
-
- Bikowski J, Rosso J, Desai A, Benes V. Double‐blind, randomized, split‐face comparison of skin tolerability of benzoyl peroxide 4% wash vs a nonmedicated synthetic detergent skin cleanser. Abstract P151. American Academy of Dermatology, 64th Annual Meeting, March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54:AB27. [CENTRAL: CN‐00602224]
Bissonnette 2009 {published data only}
-
- Bissonnette R, Bolduc C, Seite S, Nigen S, Provost N, Maari C, et al. Randomized study comparing the efficacy and tolerance of a lipophillic hydroxy acid derivative of salicylic acid and 5% benzoyl peroxide in the treatment of facial acne vulgaris. Journal of Cosmetic Dermatology 2009;8(1):19‐23. [CENTRAL: CN‐00698297; MEDLINE: ] - PubMed
Borglund 1991 {published data only}
-
- Borglund E, Kristensen B, Larsson‐Stymne B, Strand A, Veien NK, Jakobsen HB. Topical meclocycline sulfosalicylate, benzoyl peroxide, and a combination of the two in the treatment of acne vulgaris. Acta Dermato‐Venereologica 1991;71:175‐8. [CENTRAL: CN‐00075962; MEDLINE: ] - PubMed
Boutli 2003 {published data only}
-
- Boutli F, Zioga M, Koussidou T, Ioannides D, Mourellou O. Comparison of chloroxylenol 0.5% plus salicylic acid 2% cream and benzoyl peroxide 5% gel in the treatment of acne vulgaris: a randomized double‐blind study. Drugs under Experimental and Clinical Research 2003;29(3):101‐5. [CENTRAL: CN‐00464160; MEDLINE: ] - PubMed
Bowman 2005 {published data only}
-
- Bowman S, Gold M, Nasir A, Vamvakias G. Comparison of clindamycin/benzoyl peroxide, tretinoin plus clindamycin, and the combination of clindamycin/benzoyl peroxide and tretinoin plus clindamycin in the treatment of acne vulgaris: a randomized, blinded study. Journal of Drugs in Dermatology 2005;4(5):611‐8. [CENTRAL: CN‐00530277; MEDLINE: ] - PubMed
-
- Bowman S, Gold MH, Nasir A, Vamvakias G. Comparison of clindamycin/benzoyl peroxide, tretinoin plus clindamycin, and a combination of both regimens in the treatment of acne vulgaris: a randomized, blinded study. Abstract P147. American Academy of Dermatology, 64th Annual Meeting, March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB25. [CENTRAL: CN‐00602651]
Burke 1983 {published data only}
-
- Burke B, Eady EA, Cunliffe WJ. Benzoyl peroxide versus topical erythromycin in the treatment of acne vulgaris. British Journal of Dermatology 1983;108(2):199‐204. [CENTRAL: CN‐00568861; MEDLINE: ] - PubMed
Capizzi 2004 {published data only}
-
- Capizzi F, Landi F, Milani M, Amerio P. Skin tolerability and efficacy of combination therapy with hydrogen peroxide stabilized cream and adapalene gel in comparison with benzoyl peroxide cream and adapalene gel in common acne. A randomized, investigator‐masked, controlled trial. British Journal of Dermatology 2004;151(2):481‐4. [CENTRAL: CN‐00490924; MEDLINE: ] - PubMed
Cassano 2002 {published data only}
-
- Cassano N, Alessandrini G, Carrieri G, Fai D, Gabbellone M, Gravante M, et al. Treatment of mild to moderate acne vulgaris with adapalene alone or combined with other anti‐acne agents. A multicenter open trial. [Studio multicentrico in aperto sul trattamento dell'acne lieve/moderata con adapalene in monoterapia o in terapia combinata]. Giornale Italiano di Dermatologia e Venereologia 2002;137(5):369‐75. [CENTRAL: CN‐00432115]
Chalker 1983 {published data only}
-
- Chalker DK, Shalita A, Smith JG, Swann RW. A double‐blind study of the effectiveness of a 3% erythromycin and 5% benzoyl peroxide combination in the treatment of acne vulgaris. Journal of the American Academy of Dermatology 1983;9(6):933‐6. [CENTRAL: CN‐00334175; MEDLINE: ] - PubMed
Chantalat 2005 {published data only}
-
- Chantalat J, Stamatas G, Kollias N, Liu JC. Comparative efficacy of target acne lesion resolution using a novel 2% salicylic acid composition versus 10% benzoyl peroxide. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):37. [CENTRAL: CN‐00602613]
Chantalat 2006 {published data only}
-
- Chantalat J, Liu JC. Six‐week safety and efficacy evaluation of a synergistic microgel complex versus 10% benzoyl peroxide in the treatment of mild to moderate acne. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB14. [CENTRAL: CN‐00602658]
-
- Chantalat J, Luedtke K, Wiegand B, Liu JC. Significant improvement in acne quality of life by a topical treatment containing a synergistic microgel complex and 2% salicylic acid. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB21. [CENTRAL: CN‐00602548]
-
- Chantalat J, Stamatas G, Liu J C, Chen T. Treating emerging acne. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB21. [CENTRAL: CN‐00602574]
Chu 1997 {published data only}
-
- Chu A. The comparative safety and efficacy of benzoyl peroxide 5%/erythromycin 3% gel and erythromycin 4%/zinc 1.2% solution in the treatment of acne vulgaris. British Journal of Dermatology 1996;135(Suppl 47):30. [CENTRAL: CN‐00415429]
-
- Chu A, Huber FJ, Plott RT. The comparative efficacy of benzoyl peroxide 5%/erythromycin 3% gel and erythromycin 4%/zinc 1.2% solution in the treatment of acne vulgaris. British Journal of Dermatology 1997;136(2):235‐8. [CENTRAL: CN‐00137636; MEDLINE: ] - PubMed
-
- Chu A, Huber FJ, Plott RT. The comparative safety and efficacy of benzoyl peroxide 5%/erythromycin 3% gel and erythromycin 4%/zinc 1% solution in the treatment of acne vulgaris. Journal of Investigative Dermatology 1997;108(3):392. [CENTRAL: CN‐00352565] - PubMed
Coughlin 2017 {published data only}
-
- Coughlin CC, Swink SM, Horwinski J, Sfyroera G, Bugayev J, Grice EA, et al. The preadolescent acne microbiome: a prospective, randomized, pilot study investigating characterization and effects of acne therapy. Pediatric Dermatology 2017;34(6):661‐4. [CENTRAL: CN‐01432160; MEDLINE: ] - PubMed
Cunliffe 2001 {published data only}
-
- Cunliffe WJ, Glass D, Goode K, Stables GI, Boorman GC. A double‐blind investigation of the potential systemic absorption of isotretinoin, when combined with chemical sunscreens, following topical application to patients with widespread acne of the face and trunk. Acta Dermato‐Venereologica 2001;81(1):14‐7. [CENTRAL: CN‐00373092; MEDLINE: ] - PubMed
Cunliffe 2002 {published data only}
-
- Cunliffe W. A comparison of the clinical and antimicrobial efficacy of clinoxin AQ gel (1 % w/v clindamycin/5% w/v benzoyl peroxide) and 1% (w/v) clindamycin gel in the treatment of acne. British Journal of Dermatology 2000;143(Suppl 57):72. [CENTRAL: CN‐00547635]
-
- Cunliffe WJ, Holland KT, Bojar R, Levy SF. A randomized, double‐blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clinical Therapeutics 2002;24(7):1117‐33. [CENTRAL: CN‐00404978; MEDLINE: ] - PubMed
Del 2007 {published data only}
-
- Rosso J, Green L, Kempers S, Tanghetti E. A comparison of initiating treatment for acne vulgaris with 5% benzoyl peroxide/1% clindamycin gel, 0.1% adapalene gel, or both for 4 weeks then combination therapy or 0.1% adapalene gel for 8 weeks. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB22. [CENTRAL: CN‐00602643]
-
- Rosso JQ. Study results of benzoyl peroxide 5%/clindamycin 1% topical gel, adapalene 0.1% gel, and use in combination for acne vulgaris. Journal of Drugs in Dermatology 2007;6(6):616‐22. [CENTRAL: CN‐00611006; MEDLINE: ] - PubMed
Del 2009a {published data only}
-
- Rosso J, Kircik L. Comparison of the tolerability of benzoyl peroxide microsphere wash versus a gentle cleanser, when used in combination with a clindamycin and tretinoin gel: a multicenter, investigator‐blind, randomized study. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB17. [DOI: 10.1016/j.jaad.2008.11.100] - DOI
Dhawan 2013 {published data only}
-
- Dhawan SS, Gwazdauskas J. Clindamycin phosphate 1.2%‐benzoyl peroxide (5% or 2.5%) plus tazarotene cream 0.1% for the treatment of acne. Cutis; Cutaneous Medicine for the Practitioner 2013;91(2):99‐104. [CENTRAL: CN‐00869753; MEDLINE: ] - PubMed
-
- NCT01016977. A phase 4, single‐blind, randomized study to compare the tolerability and efficacy of Tazorac cream when used in combination with either Duac gel or Acanya gel for the treatment of facial acne vulgaris. clinicaltrials.gov/ct2/show/NCT01016977 (first received 20 November 2009).
Dogra 1993 {published data only}
-
- Dogra A, Sood VK, Minocha YC. Comparative evaluation of retinoic acid, benzoyl peroxide and erythromycin lotion in acne vulgaris. Indian Journal of Dermatology, Venereology and Leprology 1993;59(5):243‐6. [CENTRAL: CN‐01095331]
do Nascimento 2003 {published data only}
-
- do Nascimento LV, Guedes AC, Magalhaes GM, Faria FA, Guerra RM, Almeida F. Single‐blind and comparative clinical study of the efficacy and safety of benzoyl peroxide 4% gel (BID) and adapalene 0.1% Gel (QD) in the treatment of acne vulgaris for 11 weeks. Journal of Dermatological Treatment 2003;14(3):166‐71. [CENTRAL: CN‐00559227; MEDLINE: ] - PubMed
Draelos 2002 {published data only}
-
- Draelos ZD, Tanghetti EA. Optimizing the use of tazarotene for the treatment of facial acne vulgaris through combination therapy. Cutis; Cutaneous Medicine for the Practitioner 2002;69(2 Suppl):20‐9. [CENTRAL: CN‐00409023; MEDLINE: ] - PubMed
Draelos 2010 {published data only}
-
- Draelos ZD, Potts A, Alio Saenz AB. Randomized tolerability analysis of clindamycin phosphate 1.2%‐tretinoin 0.025% gel used with benzoyl peroxide wash 4% for acne vulgaris. Cutis; Cutaneous Medicine for the Practitioner 2010;86(6):310‐8. [CENTRAL: CN‐00770969; MEDLINE: ] - PubMed
-
- NCT00891982. A phase 3, multicenter, assessor‐blinded study of the tolerability of a topical antibiotic and retinoid used in conjunction with benzoyl peroxide wash in subjects with mild‐to‐moderate facial acne vulgaris. clinicaltrials.gov/ct2/show/NCT00891982 (first received 29 April 2009).
Dreno 2011 {published data only}
-
- Dreno B, Kaufmann R, Talarico S, Torres Lozada V, Rodriguez‐Castellanos MA, Gomez‐Flores M, et al. Combination therapy with adapalene‐benzoyl peroxide and oral lymecycline in the treatment of moderate to severe acne vulgaris: a multicentre, randomized, double‐blind controlled study. British Journal of Dermatology 2011;165(2):383‐90. [CENTRAL: CN‐00800637; MEDLINE: ] - PubMed
-
- NCT01014689. Efficacy and safety comparison of Epiduo gel associated with lymecycline 300 mg capsules versus Epiduo vehicle gel associated with lymecycline 300 mg capsules in the treatment of moderate to severe acne vulgaris (TEAM). clinicaltrials.gov/ct2/show/NCT01014689 (first received 17 November 2009).
Dreno 2016 {published data only}
-
- Dreno B, Tan J, Rivier M, Martel P, Bissonnette R. Adapalene 0.1%/benzoyl peroxide 2.5% gel reduces the risk of atrophic scar formation in moderate inflammatory acne: a split‐face randomized controlled trial. Journal of the European Academy of Dermatology and Venereology 2017;31(4):737‐42. [CENTRAL: CN‐01424729; MEDLINE: ] - PubMed
Dréno 2018 {published data only}
-
- Dréno B, Bissonnette R, Gagné‐Henley A, Barankin B, Lynde C, Kerrouche N, et al. Prevention and reduction of atrophic acne scars with adapalene 0.3%/benzoyl peroxide 2.5% gel in subjects with moderate or severe facial acne: results of a 6‐month randomized, vehicle‐controlled trial using intra‐individual comparison. American Journal of Clinical Dermatology 2018;19(2):275‐86. [CENTRAL: CN‐01572577] - PMC - PubMed
-
- NCT02735421. Adapalene 0.3% ‐ Benzoyl Peroxide 2.5% Gel and Risk of Formation of Atrophic Acne Scars (OSCAR). clinicaltrials.gov/ct2/show/NCT02735421 (first received 7 April 2016).
Dubey 2016 {published data only}
-
- Dubey A, Amane H. Comparison of efficacy and safety of adapalene and benzoyl peroxide‐clindamycin combination in the topical treatment of acne vulgaris. International Journal of Basic & Clinical Pharmacology 2016;5(5):1727‐32. [DOI: 10.18203/2319-2003.ijbcp20163207] - DOI
Dudhia 2015 {published data only}
-
- Dudhia S, Shah RB, Agrawal P, Shah A, Date S. Efficacy and safety of clindamycin gel plus either benzoyl peroxide gel or adapalene gel in the treatment of acne: a randomized open‐label study. Drugs and Therapy Perspectives 2015;31(6):208‐12. [CENTRAL: CN‐01074309]
Dunlap 1997 {published data only}
-
- Dunlap FE. An investigator blinded randomized study comparing a 3% erythromycin/5% benzoyl peroxide combination in gel versus 20% azelaic acid cream in the treatment of acne vulgaris. Abstract 126. Journal of Investigative Dermatology 1997;108(3):392. [CENTRAL: CN‐00355137]
Dunlop 1995 {published data only}
-
- Dunlop KJ, Barnetson RS. A comparative study of isolutrol versus benzoyl peroxide in the treatment of acne. Australasian Journal of Dermatology 1995;36(1):13‐5. [CENTRAL: CN‐00114537; MEDLINE: ] - PubMed
Eady 1996 {published data only}
-
- Eady EA, Bojar RA, Jones CE, Cove JH, Holland KT, Cunliffe WJ. The effects of acne treatment with a combination of benzoyl peroxide and erythromycin on skin carriage of erythromycin‐resistant propionibacteria. British Journal of Dermatology 1996;134(1):107‐13. [CENTRAL: CN‐00126809; MEDLINE: ] - PubMed
-
- Eady EA, Bojar RA, Jones CE, Cove KT, Cunliffe WJ. The effects of acne therapy with a combination of benzoyl peroxide and erythromycin on carriage of erythromycin resistant cutaneous propionibacteria. British Journal of Dermatology 1994;131(3):437‐8. [CENTRAL: CN‐00318672] - PubMed
Ede 1973 {published data only}
-
- Ede M. A double blind, comparative study of benzoyl peroxide, benzoyl peroxide chlorhydroxyquinoline, benzoyl peroxide chlorhydroxyquinoline hydrocortisone, and placebo lotions in acne. Current Therapeutic Research ‐ Clinical and Experimental 1973;15(9):624‐9. [CENTRAL: CN‐00009208; MEDLINE: ] - PubMed
Eichenfield 2011 {published data only}
-
- Eichenfield L, Alio A. Safety and efficacy of clindamycin phosphate 1.2%‐benzoyl peroxide 3% fixed dose combination gel for the treatment of acne vulgaris: a multicenter, double‐blind, parallel group, vehicle controlled study. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB10. [DOI: 10.1016/j.jaad.2011.11.049] - DOI
-
- Eichenfield LF, Alio Saenz AB. Safety and efficacy of clindamycin phosphate 1.2%‐benzoyl peroxide 3% fixed‐dose combination gel for the treatment of acne vulgaris: a phase 3, multicenter, randomized, double‐blind, active‐ and vehicle‐controlled study. Journal of Drugs in Dermatology 2011;10(12):1382‐96. [CENTRAL: CN‐00843726; MEDLINE: ] - PubMed
-
- NCT00776919. A phase 3 multicenter, randomized, double‐blind, active and vehicle‐controlled study of the safety and efficacy of a clindamycin / benzoyl peroxide gel versus clindamycin gel versus benzoyl peroxide gel versus vehicle gel in subjects with acne vulgaris. www.clinicaltrials.gov/ct2/show/NCT00776919 (first received 21 October 2008).
Eichenfield 2013 {published data only}
-
- Eichenfield L, Hebert AA, Lucky AW, Rudisill D, Sugarman J, Gold LS, et al. Treatment of acne in children aged 9 to 11 with a fixed dose combination of adapalene‐benzoyl peroxide gel. Journal of the American Academy of Dermatology 2013;68(4 Suppl 1):AB19. [CENTRAL: CN‐01028500]
-
- Eichenfield LF, Draelos Z, Lucky AW, Hebert AA, Sugarman J, Gold LS, et al. Preadolescent moderate acne vulgaris: a randomized trial of the efficacy and safety of topical adapalene‐benzoyl peroxides. Journal of Drugs in Dermatology 2013;12(6):611‐8. [CENTRAL: CN‐00914308; MEDLINE: ] - PubMed
-
- Eichenfield LF, Draelos Z, Lucky AW, Herbert AA, Sugarman J, Gold S, et al. Treatment of acne in children 9‐11 with a fixed dose combination. Pediatric Dermatology 2013;30(5):647. [CENTRAL: CN‐01025039]
-
- NCT01138735. A multi‐center, randomized, vehicle‐controlled, double‐blind study to evaluate the safety and efficacy of Epiduo® (adapalene and benzoyl peroxide) gel 0.1%/2.5% administered once daily for the treatment of subjects 9 to 11 years of age with acne vulgaris. www.clinicaltrials.gov/ct2/show/NCT01138735 (first received 4 June 2010).
Fan 1998 {published data only}
-
- Fan LH, Xu CR. A randomised controlled trial of Bimaisen (compound erythromycin and benzoyl peroxide) versus metronidazole in the treatment of acne. Journal of Clinical Dermatology 1998;27(6):385‐6. [CENTRAL: CN‐00454221]
Fang 2002 {published data only}
-
- Fang L, Fu W, Gu J, Sun J, Ma E. Efficacy and safety of benzoyl peroxide gel, 5% combined with adapalene gel, 0.1% in Chinese patients with acne vulgaris (Abstract). 20th World Congress of Dermatology, Paris, 1st to 5th July 2002. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 2):P0020. [CENTRAL: CN‐00454223]
Fleischer 2010 {published data only}
-
- Fleischer AB, Draelos ZD, Abramovits W, Pariser DM. Dapsone gel 5% in combination with adapalene gel 0.1%, benzoyl peroxide gel 4%, or vehicle gel for the treatment of acne vulgaris: a randomized, double‐blind study. Abstract P113. American Academy of Dermatology, 65th Annual Meeting, February 2‐6, 2007. Journal of the American Academy of Dermatology 2007;56(2):AB16. [CENTRAL: CN‐00616000]
-
- Fleischer AB, Shalita A, Eichenfield LF, Abramovits W, Lucky A, Garrett SCP. Dapsone gel 5% in combination with adapalene gel 0.1%, benzoyl peroxide gel 4% or moisturizer for the treatment of acne vulgaris: a 12‐week, randomized, double‐blind study. Journal of Drugs in Dermatology 2010;9(1):33‐40. [CENTRAL: CN‐00734828; MEDLINE: ] - PubMed
-
- NCT00151541. A phase 3 study to compare the safety and efficacy of 5% dapsone topical gel (DTG) twice daily in combination with once daily vehicle control, adapalene gel 0.1% or benzoyl peroxide gel 4%. www.clinicaltrials.gov/ct2/show/NCT00151541 (first received 7 September 2005).
Fu 2003 {published data only}
-
- Fu WW, Fang L, Gu J, Shun JF. Clinical efficacy and safety of 5% benzoyl peroxide gel combined with 0.1% adapalene gel in the treatment of acne vulgaris: a multicenter, randomized study. Chinese Journal of Dermatology 2003;36(6):310‐2. [CENTRAL: CN‐00484027]
Fyrand 1986 {published data only}
-
- Fyrand O, Jakobsen HB. Water‐based versus alcohol‐based benzoyl peroxide preparations in the treatment of acne vulgaris. Dermatologica 1986;172(5):263‐7. [CENTRAL: CN‐00044206; MEDLINE: ] - PubMed
Gold 2009 {published data only}
-
- Alexis AF, Johnson LA, Kerrouche N, Callender VD. A subgroup analysis to evaluate the efficacy and safety of adapalene‐benzoyl peroxide topical gel in black subjects with moderate acne. Journal of Drugs in Dermatology 2014;13(2):170‐4. [CENTRAL: CN‐00985276; MEDLINE: ] - PubMed
-
- Eichenfield LE, Jorizzo JL, Dirschka T, Taub AF, Lynde C, Graeber M, et al. Treatment of 2,453 acne vulgaris patients aged 12‐17 years with the fixed‐dose adapalene‐benzoyl peroxide combination topical gel: efficacy and safety. Journal of Drugs in Dermatology 2010;9(11):1395‐401. [CENTRAL: CN‐00888969; MEDLINE: ] - PubMed
-
- Gold LS, Tan J, Cruz‐Santana A, Papp K, Poulin Y, Schlessinger J, et al. A North American study of adapalene‐benzoyl peroxide combination gel in the treatment of acne. Cutis 2009;84:110‐6. [MEDLINE: ] - PubMed
-
- Gold LS, Tan J, Papp K, Poulin Y. A pivotal study comparing the efficacy and safety of the adapalene‐benzoyl peroxide fixed‐dose combination gel with each component and the vehicle in 1668 patients with acne vulgaris. Journal of the American Academy of Dermatology 2010;62(3 Suppl):AB16. [CENTRAL: CN‐00843732]
Gold 2010 {published data only}
-
- Brodell RT, Schlosser BJ, Rafal E, Toth D, Tyring S, Wertheimer A, et al. A fixed‐dose combination of adapalene 0.1%/BPO 2.5% allows an early and sustained improvement in quality of life and patient treatment satisfaction in severe acne. Journal of Dermatological Treatment 2012;23(1):26‐34. [CENTRAL: CN‐00860557; MEDLINE: ] - PubMed
-
- Brodell RT, Wertheimer A, Toth D, Bucher D, Rafal E, Kerrouche N, et al. Patient satisfaction with a fixed dose combination of adapalene‐benzoyl peroxide gel as part of a regimen and used alone as maintenance therapy. Journal of the American Academy of Dermatology 2013;68(4 Suppl 1):AB17. [CENTRAL: CN‐01028502]
-
- Brodell RT, Wertheimer A, Toth D, Bucher D, Rafal E, Kerrouche N, et al. Treatment with adapalene‐benzoyl peroxide improves quality of life in patients with severe acne vulgaris. Journal of the American Academy of Dermatology 2013;68(4 Suppl 1):AB19. [CENTRAL: CN‐01028499]
-
- Gold LS, Cruz A, Eichenfield L, Tan J, Jorizzo J, Kerrouche N, et al. Effective and safe combination therapy for severe acne vulgaris: a randomized, vehicle‐controlled, double‐blind study of adapalene 0.1%‐benzoyl peroxide 2.5% fixed‐dose combination gel with doxycycline hyclate 100 mg. Cutis; Cutaneous Medicine for the Practitioner 2010;85(2):94‐104. [CENTRAL: CN‐00743300; MEDLINE: ] - PubMed
-
- Gold LS, Dhuin JC, Tan J, Jorizzo J. Adapalene 0.1%/benzoyl peroxide 2.5% gel with doxycycline hyclate 100 mg tablets compared to vehicle gel with doxycycline hyclate 100 mg tablets in the treatment of severe acne vulgaris. Journal of the American Academy of Dermatology 2010;62(3 Suppl):AB13. [CENTRAL: CN‐00843733]
Gold 2016 {published data only}
-
- Alexis AF, Cook‐Bolden FE, York JP. Adapalene/benzoyl peroxide gel 0.3%/2.5%: a safe and effective acne therapy in all skin phototypes. Journal of Drugs in Dermatology 2017;16(6):574‐81. [CENTRAL: CN‐01416574; MEDLINE: ] - PubMed
-
- Gold LS, Weiss J, Rueda MJ, Liu H, Tanghetti E. Moderate and severe inflammatory acne vulgaris effectively treated with single‐agent therapy by a new fixed‐dose combination adapalene 0.3 %/benzoyl peroxide 2.5 % gel: a randomized, double‐blind, parallel‐group, controlled study. American Journal of Clinical Dermatology 2016;17(3):293‐303. [CENTRAL: CN‐01264220; MEDLINE: ] - PMC - PubMed
Gollnick 2009 {published data only}
-
- 2006‐004215‐21. A multi‐center, randomized, double‐blind, parallel‐group study to demonstrate the efficacy and safety of adapalene/benzoyl peroxide topical gel compared with adapalene topical gel, 0.1%; benzoyl peroxide topical gel, 2.5% and topical gel vehicle in subjects with acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/trial/2006‐004215‐21/DE (first received 31 October 2006).
-
- Alexis AF, Johnson LA, Kerrouche N, Callender VD. A subgroup analysis to evaluate the efficacy and safety of adapalene‐benzoyl peroxide topical gel in black subjects with moderate acne. Journal of Drugs in Dermatology 2014;13(2):170‐4. [CENTRAL: CN‐00985276; MEDLINE: ] - PubMed
-
- Eichenfield LE, Jorizzo JL, Dirschka T, Taub AF, Lynde C, Graeber M, et al. Treatment of 2,453 acne vulgaris patients aged 12‐17 years with the fixed‐dose adapalene‐benzoyl peroxide combination topical gel: efficacy and safety. Journal of Drugs in Dermatology 2010;9(11):1395‐401. [CENTRAL: CN‐00888969; MEDLINE: ] - PubMed
-
- Gollnick HP, Draelos Z, Glenn MJ, Rosoph LA, Kaszuba A, Cornelison R, et al. Adapalene‐benzoyl peroxide, a unique fixed‐dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double‐blind, controlled study in 1670 patients. British Journal of Dermatology 2009;161(5):1180‐9. [CENTRAL: CN‐00731000; MEDLINE: ] - PubMed
Goreshi 2012 {published data only}
-
- Goreshi R, Samrao A, Ehst BD. A double‐blind, randomized, bilateral comparison of skin irritancy following application of the combination acne products clindamycin/tretinoin and benzoyl peroxide/adapalene. Journal of Drugs in Dermatology 2012;11(12):1422‐6. [CENTRAL: CN‐00877482; MEDLINE: ] - PubMed
-
- Goreshi R, Samrao A, Ehst BD. Double‐blind, randomized, bilateral comparison of skin irritancy following application of the combination acne products clindamycin/tretinoin and benzoyl peroxide/adapalene. Journal of Investigative Dermatology 2012;132:S86. - PubMed
Guerra‐Tapia 2012 {published data only}
-
- Guerra‐Tapia A. Effects of benzoyl peroxide 5% clindamycin combination gel versus adapalene 0.1% on quality of life in patients with mild to moderate acne vulgaris: a randomized single‐blind study. Journal of Drugs in Dermatology 2012;11(6):714‐22. [CENTRAL: CN‐00859793; MEDLINE: ] - PubMed
-
- NCT00807014. Evaluation of quality of life, efficacy, and tolerance of Duac® gel compared to Differin® gel in the treatment of acne. www.clinicaltrials.gov/ct2/show/NCT00807014 (first received 10 December 2008).
Gupta 2003 {published data only}
-
- Gupta AK, Lynde CW, Kunynetz RA, Amin S, Choi K, Goldstein E. A randomized, double‐blind, multicenter, parallel group study to compare relative efficacies of the topical gels 3% erythromycin/5% benzoyl peroxide and 0.025% tretinoin/erythromycin 4% in the treatment of moderate acne vulgaris of the face. Journal of Cutaneous Medicine & Surgery 2003;7(1):31‐7. [CENTRAL: CN‐00435950; MEDLINE: ] - PubMed
Hayashi 2018 {published data only}
-
- 2017‐001575‐23. Clinical evaluation of efficacy at 2 weeks of Duac fixed dose combination gel in treatment of facial acne vulgaris in Japanese subjects. www.clinicaltrialsregister.eu/ctr‐search/trial/2017‐001575‐23/results (first received 21 September 2017).
-
- Hayashi N, Kurokawa I, Siakpere O, Endo A, Hatanaka T, Yamada M, et al. Clindamycin phosphate 1.2%/benzoyl peroxide 3% fixed‐dose combination gel versus topical combination therapy of adapalene 0.1% gel and clindamycin phosphate 1.2% gel in the treatment of acne vulgaris in Japanese patients: a multicenter, randomized, investigator‐blind, parallel‐group study. Journal of Dermatology 2018;45(8):951‐62. [CENTRAL: CN‐01629242] - PMC - PubMed
-
- NCT02557399. DUAC® early onset efficacy study in Japanese subjects. clinicaltrials.gov/ct2/show/NCT02557399 (first received 23 September 2015).
Hughes 1992 {published data only}
-
- Hughes BR, Norris JF, Cunliffe WJ. A double‐blind evaluation of topical isotretinoin 0.05%, benzoyl peroxide gel 5% and placebo in patients with acne. Clinical & Experimental Dermatology 1992;17(3):165‐8. [MEDLINE: ] - PubMed
Iftikhar 2009 {published data only}
-
- Iftikhar U, Aman S, Nadeem M, Kazmi AH. A comparison of efficacy and safety of topical 0.1% adapalene and 4% benzoyl peroxide in the treatment of mild to moderate acne vulgaris. Journal of Pakistan Association of Dermatologists 2009;19(3):141‐5. [www.jpad.com.pk/index.php/jpad/article/view/530]
Jackson 2010 {published data only}
-
- Jackson JM, Fu JJ, Almekinder JL. A randomized, investigator‐blinded trial to assess the antimicrobial efficacy of a benzoyl peroxide 5%/clindamycin phosphate 1% gel compared with a clindamycin phosphate 1.2%/tretinoin 0.025% gel in the topical treatment of acne vulgaris. Journal of Drugs in Dermatology 2010;9(2):131‐6. [CENTRAL: CN‐00743909; MEDLINE: ] - PubMed
-
- NCT00841776. Comparative antimicrobial efficacy of two topical acne therapies for the treatment of facial acne. www.clinicaltrials.gov/ct2/show/NCT00841776 (first received 10 February 2009).
Jaffe 1989 {published data only}
-
- Jaffe GV, Grimshaw JJ, Constad D. Benzoyl peroxide in the treatment of acne vulgaris: a double‐blind, multi‐centre comparative study of 'Quinoderm' cream and 'Quinoderm' cream with hydrocortisone versus their base vehicle alone and a benzoyl peroxide only gel preparation. Current Medical Research & Opinion 1989;11(7):453‐62. [CENTRAL: CN‐00062342; MEDLINE: ] - PubMed
Jawade 2016 {published data only}
-
- Jawade SA, Saigaonkar VA, Kondalkar AR. Efficacy and tolerability of adapalene 0.1%‐benzoyl peroxide 2.5% combination gel in treatment of acne vulgaris in Indian patients: a randomized investigator‐blind controlled trial. Iranian Journal of Dermatology 2016;19(4):105‐12. [CENTRAL: CN‐01405922]
Ji 2000 {published data only}
-
- Ji S, Tu P, Li GQ, Liu LL, Chen XX, Zhu XJ. A comparison of 10% benzoyl peroxide cream and 5% benzoyl peroxide gel in the treatment of acne vulgaris. Chinese Journal of Clinical Pharmacology 2000;16(1):15‐7. [CENTRAL: CN‐00602357]
Jones 2002 {published data only}
-
- Jones T, Mark L, Monroe E, Weiss J, Levy S. A multicentre, double‐blind, parallel‐group study to evaluate 3% erythromycin/5% benzoyl peroxide dual‐pouch pack for acne vulgaris. Clinical Drug Investigation 2002;22(7):455‐62. [CENTRAL: CN‐00405023]
Kabir 2018 {published data only}
-
- Kabir M, Sadiq S, Raza A, Kanwal S, Tanvir T. Comparison of efficacy of adapalene (0.1% gel) monotherapy vs adapalene (0.1%) plus benzyl peroxide (2.5%) combination therapy for treatment of mild to moderate acne vulgaris. Pakistan Journal of Medical and Health Sciences 2018;12(2):587‐9.
Kaur 2015 {published data only}
Kawashima 2014 {published data only}
-
- Kawashima M, Hashimoto H, Alio Saenz AB, Ono M, Yamada M. Is benzoyl peroxide 3% topical gel effective and safe in the treatment of acne vulgaris in Japanese patients? A multicenter, randomized, double‐blind, vehicle‐controlled, parallel‐group study. Journal of Dermatology 2014;41(9):795‐801. [CENTRAL: CN‐01254201; MEDLINE: ] - PubMed
-
- NCT01400932. Study STF115288, a clinical confirmation study of GI148512 in the treatment of acne vulgaris in Japanese subjects. www.clinicaltrials.gov/ct2/show/NCT01400932 (first received 21 July 2011).
Kawashima 2015 {published data only}
-
- Kawashima M, Hashimoto H, Alio Saenz AB, Ono M, Yamada M. Clindamycin phosphate 12%‐benzoyl peroxide 30% fixed‐dose combination gel has an effective and acceptable safety and tolerability profile for the treatment of acne vulgaris in Japanese patients: a phase III, multicentre, randomised, single‐blinded, active‐controlled, parallel‐group study. British Journal of Dermatology 2015;172(2):494‐503. [CENTRAL: CN‐01115087] - PubMed
-
- Kawashima M, Yamada M, Parish C. Clindamycin 1%/benzoyl peroxide 3% gel, a new topical combination product, is effective in Japanese patients with acne vulgaris. Journal of Investigative Dermatology 2013;133:S160. [CENTRAL: CN‐01026775]
Kawashima 2017a {published data only}
-
- Kawashima M, Nagare T, Katsuramaki T. Open‐label, randomized, multicenter, phase III study to evaluate the safety and efficacy of benzoyl peroxide gel in long‐term use in patients with acne vulgaris: a secondary publication. Journal of Dermatology 2017; Vol. 44, issue 6:635‐43. [CENTRAL: CN‐01459134; MEDLINE: ] - PMC - PubMed
-
- Morimoto H, Kikukawa Y, Murakami N. Pharmacological profiles and clinical effects of benzoyl peroxide gel as treatments for acne vulgaris. Nippon Yakurigaku Zasshi ‐ Folia Pharmacologica Japonica 2015;146(4):225‐32. [PUBMED: 26656967] - PubMed
Kawashima 2017b {published data only}
-
- Kawashima M, Sato S, Furukawa F, Matsunaga K, Akamatsu H, Igarashi A, et al. Twelve‐week, multicenter, placebo‐controlled, randomized, double‐blind, parallel‐group, comparative phase II/III study of benzoyl peroxide gel in patients with acne vulgaris: a secondary publication. Journal of Dermatology 2017; Vol. 44, issue 7:774‐82. [CENTRAL: CN‐01458975] - PMC - PubMed
-
- Morimoto H, Kikukawa Y, Murakami N. Pharmacological profiles and clinical effects of benzoyl peroxide gel as treatments for acne vulgaris. Nippon Yakurigaku Zasshi ‐ Folia Pharmacologica Japonica 2015;146(4):225‐32. [PUBMED: 26656967] - PubMed
Ko 2009 {published data only}
-
- Ko HC, Song M, Seo SH, Oh CK, Kwon KS, Kim MB. Prospective, open‐label, comparative study of clindamycin 1%/benzoyl peroxide 5% gel with adapalene 0.1% gel in Asian acne patients: efficacy and tolerability. Journal of the European Academy of Dermatology & Venereology 2009;23(3):245‐50. [CENTRAL: CN‐00697965; MEDLINE: ] - PubMed
Korkut 2005 {published data only}
-
- Korkut C, Piskin S. Benzoyl peroxide, adapalene, and their combination in the treatment of acne vulgaris. Journal of Dermatology 2005;32(3):169‐73. [CENTRAL: CN‐00512006] - PubMed
Langner 2007 {published data only}
-
- Langner A, Sheehan‐Dare R, Layton A. A randomized, single‐blind comparison of topical clindamycin + benzoyl peroxide (Duac) and erythromycin + zinc acetate (Zineryt) in the treatment of mild to moderate facial acne vulgaris. Journal of the European Academy of Dermatology & Venereology 2007;21(3):311‐9. [CENTRAL: CN‐00586947; MEDLINE: ] - PubMed
Langner 2008 {published data only}
-
- Langner A, Chu A, Goulden V, Ambroziak M. A randomized, single‐blind comparison of topical clindamycin + benzoyl peroxide and adapalene in the treatment of mild to moderate facial acne vulgaris. British Journal of Dermatology 2008;158(1):122‐9. [CENTRAL: CN‐00628563; MEDLINE: ] - PubMed
Leyden 2001a {published data only}
-
- Ellis CN, Leyden J, Katz HI, Goldfarb MT, Hickman J, Jones TM. Therapeutic studies with a new combination benzoyl peroxide/clindamycin topical gel in acne vulgaris. Cutis; Cutaneous Medicine for the Practitioner 2001;67(2 Suppl):13‐20. [CENTRAL: CN‐00520424; MEDLINE: ] - PubMed
-
- Leyden J, et al. Treatment of acne vulgaris with a combination benzoyl peroxide/clindamycin topical gel Abstract P‐013. 8th Congress of the European Academy of Dermatology and Venereology. Amsterdam, The Netherlands, 29 Sept‐3 October 1999. Journal of the European Academy of Dermatology and Venereology 1999;12(Suppl 2):S144. [CENTRAL: CN‐00478651]
-
- Leyden JJ, Berger RS, Dunlap FE, Ellis CN, Connolly MA, Levy SF. Comparison of the efficacy and safety of a combination topical gel formulation of benzoyl peroxide and clindamycin with benzoyl peroxide, clindamycin and vehicle gel in the treatments of acne vulgaris. American Journal of Clinical Dermatology 2001;2(1):33‐9. [CENTRAL: CN‐00363426; MEDLINE: ] - PubMed
Leyden 2001b1 {published data only}
-
- Leyden J, Levy S. The development of antibiotic resistance in Propionibacterium acnes. Cutis; Cutaneous Medicine for the Practitioner 2001;67(2 Suppl):21‐4. [CENTRAL: CN‐00509691; MEDLINE: ] - PubMed
Leyden 2001b2 {published data only}
-
- Leyden J, Levy S. The development of antibiotic resistance in Propionibacterium acnes. Cutis; Cutaneous Medicine for the Practitioner 2001;67(2 Suppl):21‐4. [CENTRAL: CN‐00509691; MEDLINE: ] - PubMed
Lookingbill 1997 {published data only}
-
- Lookingbill DP, Chalker DK, Lindholm JS, Katz HI, Kempers SE, Huerter CJ, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double‐blind investigations. Journal of the American Academy of Dermatology 1997;37(4):590‐5. [CENTRAL: CN‐00144503; MEDLINE: ] - PubMed
-
- Lookingbill DP, Challen DK, Lindholm JS. Treatment of acne with a combination of clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined result of two double blind investigations (Abstract no. 1). Indian Journal of Dermatology, Venerology and Leprology 1995;61:398. - PubMed
Lyons 1978 {published data only}
-
- Lyons RE. Comparative effectiveness of benzoyl peroxide and tretinoin in acne vulgaris. International Journal of Dermatology 1978;17(3):246‐51. [CENTRAL: CN‐00018160; MEDLINE: ] - PubMed
Makino 2015 {published data only}
-
- Makino E, Mehta R. A nondrying acne regimen provides rapid reduction in erythema and efficacy comparable to adapalene/benzoyl peroxide 0.1%/2.5% combination. Journal of the American Academy of Dermatology 2015;72(5 Suppl 1):AB4. [CENTRAL: CN‐01088986]
Marazzi 2002 {published data only}
-
- Marazzi P, Boorman GC, Donald AE, Davies HD. Clinical evaluation of Double Strength Isotrexin versus Benzamycin in the topical treatment of mild to moderate acne vulgaris. Journal of Dermatological Treatment 2002;13(3):111‐7. [CENTRAL: CN‐00409983; MEDLINE: ] - PubMed
Milani 2003 {published data only}
-
- Milani M, Bigardi A, Zavattarelli M. Efficacy and safety of stabilised hydrogen peroxide cream (Crystacide) in mild‐to‐moderate acne vulgaris: a randomised, controlled trial versus benzoyl peroxide gel. Current Medical Research & Opinion 2003;19(2):135‐8. [CENTRAL: CN‐00437298; MEDLINE: ] - PubMed
Mills 1986 {published data only}
-
- Mills Jr OH, Kligman AM, Pochi P, Comite H. Comparing 2.5%, 5%, and 10% benzoyl peroxide on inflammatory acne vulgaris. International Journal of Dermatology 1986;25(10):664‐7. [CENTRAL: CN‐00046261; MEDLINE: ] - PubMed
Miyachi 2016 {published data only}
-
- Miyachi Y, Mizzi F, Mita T, Bai L, Ikoma A. Efficacy and safety of a fixed dose combination gel of adapalene 0.1% and benzoyl peroxide 2.5% in Japanese patients with acne vulgaris ‐ a multicenter, randomized, double‐blinded, active‐controlled, parallel group phase III study. [Japanese]. Skin Research 2016;15(4):278‐93.
-
- NCT02073448. Efficacy and safety study of GK530G versus CD0271 0.1% gel and CD1579 2.5% gel in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT02073448 (first received 20 February 2014).
NCT00713609 {published data only}
-
- 2015‐004900‐44. A multi‐center randomized double blind vehicle‐controlled, phase 2 study of the safety and efficacy of benzoyl peroxide/clindamycin gel and tazarotene cream when used in combination in the treatment of acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/trial/2015‐004900‐44/results (results published 23 March 2017).
-
- NCT00713609. Safety and efficacy study of clindamycin/benzoyl peroxide/tazarotene cream in subjects with acne. clinicaltrials.gov/ct2/show/NCT00713609 (first received 7 July 2008).
NCT00787943 {published data only}
-
- NCT00787943. Study of two different 10.0% benzoyl peroxide creams for mild to moderate acne vulgaris. www.clinicaltrials.gov/ct2/show/NCT00787943 (first received 6 November 2008).
NCT01044264 {published data only}
-
- NCT01044264. Clinical study between two 1% clindamycin/5% benzoyl peroxide topical gel formulations. https://clinicaltrials.gov/ct2/show/NCT01044264 (first received 4 January 2010).
NCT01138514 {published data only}
-
- NCT01138514. Clinical study between two clindamycin 1%/benzoyl peroxide 5% topical gels. clinicaltrials.gov/ct2/show/NCT01138514 (first received 4 June 2010).
NCT01231334 {published data only}
-
- NCT01231334. A study comparing Aczone® plus Differin® versus Duac® plus Differin® in patients with severe facial acne. clinicaltrials.gov/ct2/show/NCT01231334 (first received 28 October 2010).
NCT01522456 {published data only}
-
- NCT01522456. Split‐face tolerability comparison between adapalene‐benzoyl peroxide gel versus tretinoin gel. www.clinicaltrials.gov/ct2/show/NCT01522456 (first received 23 January 2012).
NCT02073461 {published data only}
-
- NCT02073461. Efficacy and safety study of 2 different concentrations of CD1579 gels versus vehicle in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT02073461 (first received 20 February 2014).
NCT02465632 {published data only}
-
- NCT02465632. To study generic clindamycin 1%/benzoyl peroxide 5% topical gel (Glenmark Generics, Ltd) in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT02465632 (first received 4 June 2015).
Ozgen 2013 {published data only}
-
- Ozgen ZY, Gurbuz O. A randomized, double‐blind comparison of nadifloxacin 1% cream alone and with benzoyl peroxide 5% lotion in the treatment of mild to moderate facial acne vulgaris. Marmara Medical Journal 2013;26(1):243‐6. [CENTRAL: CN‐00912356]
Packman 1996 {published data only}
-
- Packman A. A controlled, randomized study comparing the efficacy of a 3% erythromycin and 5% benzoyl peroxide combination gel with clindamycin phosphate lotion for the treatment of acne vulgaris. Abstract P138. Journal of the European Academy of Dermatology and Venereology 1995;5:S152. [CENTRAL: CN‐00602580]
-
- Packman AM, Brown RH, Dunlap FE, Kraus SJ, Webster GF. Treatment of acne vulgaris: combination of 3% erythromycin and 5% benzoyl peroxide in a gel compared to clindamycin phosphate lotion. International Journal of Dermatology 1996;35(3):209‐11. [CENTRAL: CN‐00125217; MEDLINE: ] - PubMed
Papageorgiou 2000 {published data only}
-
- Papageorgiou PP, Chu AC. Chloroxylenol and zinc oxide containing cream (Nels cream) vs. 5% benzoyl peroxide cream in the treatment of acne vulgaris. A double‐blind, randomized, controlled trial. Clinical & Experimental Dermatology 2000;25(1):16‐20. [CENTRAL: CN‐00275100; MEDLINE: ] - PubMed
Pariser 2014 {published data only}
-
- Alexis AF, Cook‐Bolden F, Lin T, Bulley B. Treatment of moderate‐to‐severe acne vulgaris in a Hispanic population: a post‐hoc analysis of the efficacy and tolerability of clindamycin 1.2%/benzoyl peroxide 3.75% gel. Journal of Clinical and Aesthetic Dermatology 2017;10(6):36‐43. [CENTRAL: CN‐01471496; MEDLINE: ] - PMC - PubMed
-
- Gold MH, Korotzer A. Sub‐group analyses from a trial of a fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% gel for the treatment of moderate‐to‐severe acne vulgaris. Journal of Clinical and Aesthetic Dermatology 2015; Vol. 8, issue 12:22‐6. [CENTRAL: CN‐01162058; MEDLINE: ] - PMC - PubMed
-
- Harper JC. The efficacy and tolerability of a fixed combination clindamycin (1.2%) and benzoyl peroxide (3.75%) aqueous gel in patients with facial acne vulgaris: gender as a clinically relevant outcome variable. Journal of Drugs in Dermatology 2015;14(4):381‐4. [CENTRAL: CN‐01070825; MEDLINE: ] - PubMed
-
- Pariser DM, Rich P, Cook‐Bolden FE, Korotzer A. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% for the once daily treatment of moderate‐to‐severe acne vulgaris: assessment of safety in 498 patients. Journal of Clinical and Aesthetic Dermatology 2015;8(5 Suppl 1):S6.
Prince 1982 {published data only}
-
- Prince RA, Harris JM, Maroc JA. Comparative trial of benzoyl peroxide versus benzoyl peroxide with urea in inflammatory acne. Cutis; Cutaneous Medicine for the Practitioner 1982;29(6):638‐40, 644‐5. [CENTRAL: CN‐00028602; MEDLINE: ] - PubMed
Schaller 2016 {published data only}
-
- 2013‐004158‐81. A multi‐centre, single‐blind, parallel group, clinical evaluation of the efficacy and safety of clindamycin 1%/benzoyl peroxide 3% and azelaic acid 20% in the topical treatment of mild to moderate acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/trial/2013‐004158‐81/results (results published 15 March 2016).
-
- NCT02058628. Comparison of the efficacy and safety of clindamycin + benzoyl peroxide formulation with azelaic acid formulation in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT02058628 (first received 6 February 2014).
-
- Schaller M, Sebastian M, Ress C, Seidel D, Hennig M. A multicentre, randomized, single‐blind, parallel‐group study comparing the efficacy and tolerability of benzoyl peroxide 3%/clindamycin 1% with azelaic acid 20% in the topical treatment of mild‐to‐moderate acne vulgaris. Journal of the European Academy of Dermatology and Venereology 2016; Vol. 30, issue 6:966‐73. [CENTRAL: CN‐01369030; MEDLINE: ] - PubMed
Schmidt 1988 {published data only}
-
- Schmidt JB, Neumann R, Fanta D, Raab W. 1 percent clindamycin phosphate solution versus 5 percent benzoyl peroxide gel in papulopustular acne. Zeitschrift fur Hautkrankheiten 1988;63(5):374‐6. [PUBMED: 2970159] - PubMed
Shafiq 2014 {published data only}
-
- Shafiq Y, Naqvi BS, Rizwani GH, Usman M, Shah BA, Aslam M, et al. Anti‐acne activity of Casuarina equisetifolia bark extract: a randomized clinical trial. Bangladesh Journal of Pharmacology 2014;9(3):337‐41. [CENTRAL: CN‐00998392]
Shahid 1996 {published data only}
-
- Shahid J, Kamran T. Tretinoin cream versus benzoyl peroxide(10%) gel in the tropical treatment of mild acne vulgaris. Biomedica 1996;12(1):22‐4. [CENTRAL: CN‐00777740]
Shalita 1989 {published data only}
-
- Shalita AR. Comparison of a salicylic acid cleanser and a benzoyl peroxide wash in the treatment of acne vulgaris. Clinical Therapeutics 1989;11(2):264‐7. [CENTRAL: CN‐00185376; MEDLINE: ] - PubMed
Shalita 2003 {published data only}
-
- Shalita AR, Rafal ES, Anderson DN, Yavel R, Landow S, Lee WL. Compared efficacy and safety of tretinoin 0.1% microsphere gel alone and in combination with benzoyl peroxide 6% cleanser for the treatment of acne vulgaris. Cutis; Cutaneous Medicine for the Practitioner 2003;72(2):167‐72. [CENTRAL: CN‐00457639; MEDLINE: ] - PubMed
Shwetha 2014 {published data only}
-
- Shwetha H, Geetha A. A comparative study of efficacy and safety of combination of topical 1% clindamycin and 0.1% adapalene with 1% clindamycin and 2.5% benzoyl peroxide in mild to moderate acne in a tertiary care hospital. Indian Journal of Pharmacology 2013;45:S19. [CENTRAL: CN‐01057398]
-
- Shwetha H, Geetha A, Revathi TN. A comparative study of efficacy and safety of combination of topical 1% clindamycin and 0.1% adapalene with 1% clindamycin and 2.5% benzoyl peroxide in mild to moderate acne at a tertiary care hospital. Journal of Chemical and Pharmaceutical Research 2014;6(2):736‐41. [CENTRAL: CN‐00986447]
Sklar 1996 {published data only}
-
- Sklar JL, Jacobson C, Rizer R, Gans EH. Evaluation of triaz 10% gel and benzamycin in acne vulgaris. Journal of Dermatological Treatment 1996;7(3):147‐52. [CENTRAL: CN‐00173449]
Smith 1980 {published data only}
-
- Smith EB, Padilla RS, McCabe JM, Becker LE. Benzoyl peroxide lotion (20 percent) in acne. Cutis; Cutaneous Medicine for the Practitioner 1980;25(1):90‐2. [CENTRAL: CN‐00269336; MEDLINE: ] - PubMed
Smith 2006 {published data only}
-
- Smith S, Kempers S. Multicenter, randomized, evaluator‐blinded, parallel group comparison study of the safety and efficacy of microentrapped benzoyl peroxide cream versus benzoyl peroxide gel in mild to moderate acne vulgaris. Abstract P165. American Academy of Dermatology 64th Annual Meeting, March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB30. [CENTRAL: CN‐00602161]
-
- Smith SR, Kempers S. A study of 5.5% benzoyl peroxide microsphere cream versus 6% benzoyl peroxide gel in the treatment of acne vulgaris. Cosmetic Dermatology 2006;19(8):537‐42. [CENTRAL: CN‐00641810]
Stinco 2007 {published data only}
-
- Stinco G, Bragadin G, Trotter D, Pillon B, Patrone P. Relationship between sebostatic activity, tolerability and efficacy of three topical drugs to treat mild to moderate acne. Journal of the European Academy of Dermatology and Venereology 2007;21(3):320‐5. [CENTRAL: CN‐00586948; MEDLINE: ] - PubMed
Study 152 {published data only}
-
- FDA, Center for Drug Evaluation and Research. Study 152. Medical Review of NDA 50‐741. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/50‐741_duac%20to....
Study 156 {published data only}
-
- FDA, Center for Drug Evaluation and Research. Study 156. Medical Review of NDA 50‐741. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/50‐741_duac%20to....
Swinyer 1988 {published data only}
-
- Swinyer LJ, Baker MD, Swinyer TA, Mills OH Jr. A comparative study of benzoyl peroxide and clindamycin phosphate for treating acne vulgaris. British Journal of Dermatology 1988;119(5):615‐22. [CENTRAL: CN‐00057230; MEDLINE: ] - PubMed
Tabasum 2014 {published data only}
-
- Tabasum H, Ahmad T, Anjum F, Rehman H. The effect of Unani antiacne formulation (Zimade Muhasa) on acne vulgaris: a single blind, randomized, controlled clinical trial. Journal of Pakistan Association of Dermatologists 2014;24(4):319‐26. [CENTRAL: CN‐01075653]
Tanghetti 2006 {published data only}
-
- Tanghetti E, Abramovits W, Solomon B, Loven K, Shalita A. Tazarotene versus tazarotene plus clindamycin/benzoyl peroxide in the treatment of acne vulgaris: a multicenter, double‐blind, randomized parallel‐group trial. Journal of Drugs in Dermatology 2006;5(3):256‐61. [CENTRAL: CN‐00563788; MEDLINE: ] - PubMed
Thiboutot 2002 {published data only}
-
- Thiboutot D, Jarratt M, Rich P, Rist T, Rodriguez D, Levy S. A randomized, parallel, vehicle‐controlled comparison of two erythromycin/benzoyl peroxide preparations for acne vulgaris. Clinical Therapeutics 2002;24(5):773‐85. [CENTRAL: CN‐00396107; MEDLINE: ] - PubMed
Thiboutot 2007 {published data only}
-
- Alexis AF, Johnson LA, Kerrouche N, Callender VD. A subgroup analysis to evaluate the efficacy and safety of adapalene‐benzoyl peroxide topical gel in black subjects with moderate acne. Journal of Drugs in Dermatology 2014;13(2):170‐4. [CENTRAL: CN‐00985276; MEDLINE: ] - PubMed
-
- Eichenfield LE, Jorizzo JL, Dirschka T, Taub AF, Lynde C, Graeber M, et al. Treatment of 2,453 acne vulgaris patients aged 12‐17 years with the fixed‐dose adapalene‐benzoyl peroxide combination topical gel: efficacy and safety. Journal of Drugs in Dermatology 2010;9(11):1395‐401. [CENTRAL: CN‐00888969; MEDLINE: ] - PubMed
-
- Gold LS, Baldwin H, Rueda MJ, Kerrouche N, Dreno B. Adapalene‐benzoyl peroxide gel is efficacious and safe in adult female acne, with a profile comparable to that seen in teen‐aged females. Journal of Clinical and Aesthetic Dermatology 2016;9(7):23‐9. [CENTRAL: CN‐01197640; MEDLINE: ] - PMC - PubMed
-
- Gold MH, Korotzer A. Sub‐group analyses from a trial of a fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% gel for the treatment of moderate‐to‐severe acne vulgaris. Journal of Clinical and Aesthetic Dermatology 2015;8(12):22‐6. [CENTRAL: CN‐01162058; MEDLINE: ] - PMC - PubMed
-
- Thiboutot DM, Weiss J, Bucko A, Eichenfield L, Jones T, Clark S, et al. Adapalene‐benzoyl peroxide, a fixed‐dose combination for the treatment of acne vulgaris: results of a multicenter, randomized double‐blind, controlled study. Journal of the American Academy of Dermatology 2007;57(5):791‐9. [CENTRAL: CN‐00610848; MEDLINE: ] - PubMed
Thiboutot 2008 {published data only}
-
- Cook‐Bolden F, Chen D, Eichenfield L, Stein‐Gold L. Managing moderate to severe acne in adolescents: benefits of a fixed combination clindamycin phosphate (1.2%) and low concentration benzoyl peroxide (2.5%) aqueous gel in a subpopulation of 1755 subjects. P740. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB22. [DOI: 10.1016/j.jaad.2008.11.121] - DOI
-
- Cook‐Bolden FE. Treatment of moderate to severe acne vulgaris in a Hispanic population: a post‐hoc analysis of efficacy and tolerability of clindamycin phosphate 1.2%/benzoyl peroxide 2.5% gel. Journal of Drugs in Dermatology 2012;11(4):455‐9. [CENTRAL: CN‐00880750; MEDLINE: ] - PubMed
-
- Eichenfield LF, Krakowski AC. Moderate to severe acne in adolescents with skin of color: benefits of a fixed combination clindamycin phosphate 1.2% and benzoyl peroxide 2.5% aqueous gel. Journal of Drugs in Dermatology 2012;11(7):818‐24. [CENTRAL: CN‐00879677; MEDLINE: ] - PubMed
-
- Gold MH, Korotzer A. Sub‐group analyses from a trial of a fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 3.75% gel for the treatment of moderate‐to‐severe acne vulgaris. Journal of Clinical and Aesthetic Dermatology 2015;8(12):22‐6. [CENTRAL: CN‐01162058; MEDLINE: ] - PMC - PubMed
-
- Stein‐Gold L, Chen D, Cook‐Bolden F, Baldwin H. Efficacy of a once‐daily fixed combination clindamycin phosphate (1.2%) and low concentration benzoyl peroxide (2.5%) aqueous gel across a broad patient population with moderate to severe acne. Journal of the American Academy of Dermatology 2010;62(3 Suppl):AB15. [CENTRAL: CN‐00843902]
Tirado‐Sanchez 2009 {published data only}
-
- Tirado‐Sanchez A, Ponce‐Olivera RM. Efficacy and tolerance of superoxidized solution in the treatment of mild to moderate inflammatory acne. A double‐blinded, placebo‐ controlled, parallel‐group, randomized, clinical trial. Journal of Dermatological Treatment 2009;20(5):289‐92. [CENTRAL: CN‐00743204; MEDLINE: ] - PubMed
Tschen 2001 {published data only}
-
- Ellis CN, Leyden J, Katz HI, Goldfarb MT, Hickman J, Jones TM, et al. Therapeutic studies with a new combination benzoyl peroxide/clindamycin topical gel in acne vulgaris. Cutis; Cutaneous Medicine for the Practitioner 2001;67(2 Suppl):13‐20. [CENTRAL: CN‐00520424; MEDLINE: ] - PubMed
-
- Tschen E, et al. A combination benzoyl peroxide/clindamycin topical gel for the treatment of acne vulgaris. Abstract P‐029. 8th Congress of the European Academy of Dermatology and Venereology. Amsterdam, The Netherlands, 29 Sept‐3 October 1999. Journal of the European Academy of Dermatology and Venereology 1999;12(Suppl 2):S150. [CENTRAL: CN‐00478786]
-
- Tschen EH, Katz HI, Jones TM, Monroe EW, Kraus SJ, Connolly MA, et al. A combination benzoyl peroxide and clindamycin topical gel compared with benzoyl peroxide, clindamycin phosphate, and vehicle in the treatment of acne vulgaris. Cutis; Cutaneous Medicine for the Practitioner 2001;67(2):165‐9. [CENTRAL: CN‐00346768; MEDLINE: ] - PubMed
Tucker 1984 {published data only}
-
- Tucker SB, Tausend R, Cochran R, Flannigan SA. Comparison of topical clindamycin phosphate, benzoyl peroxide, and a combination of the two for the treatment of acne vulgaris. British Journal of Dermatology 1984;110(4):487‐92. [CENTRAL: CN‐00568865; MEDLINE: ] - PubMed
-
- Tucker SB, Tausend T, Cochran R. Comparison of topical clindamycin phosphate, benzoyl peroxide and a combination of the two, for the treatment of acne vulgaris [abstract no:1]. Indian Journal of Dermatology, Venerology and Leprology 1990;56:179. [CENTRAL: CN‐00692634] - PubMed
Tung 2014 {published data only}
-
- Tung R, Berardesca E, Dall'Oglio F, Micali G, Sinagra J, Nodzenski M, et al. Novel over‐the‐counter hydrogen peroxide based acne kit in treating acne: randomized, controlled, multicenter study of a hydrogen peroxide‐based acne system versus the benzoyl peroxide‐based acne system in the treatment of mild to moderate acne vulgaris. P8511. Journal of the American Academy of Dermatology 2014;70(5 Suppl 1):AB9. [www.jaad.org/article/S0190‐9622(14)00039‐5/pdf]
Vasarinsh 1969 {published data only}
-
- Vasarinsh P. Benzoyl peroxide‐sulfur lotions in acne vulgaris ‐ a controlled study. Cutis; Cutaneous Medicine for the Practitioner 1969;5(1):65‐9. [CENTRAL: CN‐00269401]
Wang 2003 {published data only}
-
- Wang AP, Tu P, Ji S Z, Wu Y, Shen Y, Zhu XJ. Clinical efficacy of benzoyl peroxide gel with different concentrations in acne vulgaris. Chinese Journal of Dermatology 2003;36(6):313‐5. [CENTRAL: CN‐00486355]
Weiss 2015 {published data only}
-
- Stein Gold L, Werschler WP, Mohawk J. Adapalene/benzoyl peroxide gel 0.3%/2.5%: effective acne therapy regardless of age or gender. Journal of Drugs in Dermatology 2017;16(6):582‐9. [CENTRAL: CN‐01458199; MEDLINE: ] - PubMed
-
- Weiss J, Gold LS, Rueda MJ, Tanghetti E. Adapalene 0.3%/benzoyl peroxide 2.5% gel for the treatment of severe inflammatory acne: a randomized, double‐blind, parallel‐group, controlled study. Pediatric Dermatology 2017;34:S103. [CENTRAL: CN‐01399571]
-
- Weiss J, Gold LS, Tanghetti E, Bouvresse S, Yao M, Dujols C, et al. Efficacy and safety of adapalene 0.3%/benzoyl peroxide 2.5% topical gel in moderate and severe acne vulgaris. Journal of the American Academy of Dermatology 2015;72(5 Suppl 1):AB7. [CENTRAL: CN‐01088984]
-
- Weiss J, Gold LS, Tanghetti E, Dujols C, Rueda MJ. Efficacy and safety of adapalene 0.3%/benzoyl peroxide 2.5% topical gel in moderate and severe acne vulgaris. Journal of the American Academy of Dermatology 2017; Vol. 34:S31. [CENTRAL: CN‐01399572]
-
- Weiss J, Stein Gold L, Leoni M, Rueda Mj, Liu H, Tanghetti E. Customized single‐agent therapy management of severe inflammatory acne: a randomized, double‐blind, parallel‐group controlled study of a new treatment ‐ adapalene 0.3%‐benzoyl peroxide 2.5% gel. Journal of Drugs in Dermatology 2015;14(12):1427‐35. [CENTRAL: CN‐01200535; MEDLINE: ] - PubMed
Xu 2016 {published data only}
-
- 2015‐004909‐16. A multicentre, randomized, assessor‐blind, comparator‐controlled, parallel‐group clinical trial to establish the efficacy and safety of Duac™ (1% clindamycin as clindamycin phosphate and 5% benzoyl peroxide) once daily gel compared with clindamycin phosphate gel (1% clindamycin as clindamycin phosphate) twice daily in the treatment of mild to moderate acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/trial/2015‐004909‐16/results (results published 29 December 2016).
-
- NCT01915732. Efficacy and safety of Duac™ compared with clindamycin phosphate gel in the treatment of mild to moderate acne vulgaris. clinicaltrials.gov/ct2/show/NCT01915732 (first received 5 April 2013).
-
- Xu J, Lu Q, Huang J, Sun Q, Hao F, Gu J, et al. A multicenter, randomized, single‐blind study of topical clindamycin 1%/benzoyl peroxide 5% once‐daily fixed dose combination gel (Duac) vs. clindamycin 1% twice‐daily gel in treatment of Chinese acne vulgaris patients. Journal of Investigative Dermatology 2015;135(Suppl 2):S34, 192. [CENTRAL: CN‐01130701]
-
- Xu JH, Lu QJ, Huang JH, Hao F, Sun QN, Fang H, et al. A multicentre, randomized, single‐blind comparison of topical clindamycin 1%/benzoyl peroxide 5% once‐daily gel versus clindamycin 1% twice‐daily gel in the treatment of mild to moderate acne vulgaris in Chinese patients. Journal of the European Academy of Dermatology and Venereology 2016;30(7):1176‐82. [CENTRAL: CN‐01178389; MEDLINE: ] - PubMed
Yong 1979 {published data only}
-
- Yong CC. Benzoyl peroxide gel therapy in acne in Singapore. International Journal of Dermatology 1979;18(6):485‐8. [CENTRAL: CN‐00560673; MEDLINE: ] - PubMed
Zeichner 2013 {published data only}
-
- Zeichner JA, Wong V, Linkner RV, Haddican M. Efficacy and safety of tretinoin 0.025%/clindamycin phosphate 1.2% gel in combination with benzoyl peroxide 6% cleansing cloths for the treatment of facial acne vulgaris. Journal of Drugs in Dermatology 2013;12(3):277‐82. [CENTRAL: CN‐00876866; MEDLINE: ] - PubMed
Zeng 2012 {published data only}
-
- Zeng X, Liu W L, Zhao T. Effects of Chinese medical facial mask comprehensive therapy in treating acne vulgaris. Zhongguo Zhong Xi Yi Jie He za Zhi [Chinese Journal of Integrated Traditional and Western Medicine] 2012;32(5):624‐7. [CENTRAL: CN‐00961274; MEDLINE: ] - PubMed
Zhao 2001 {published data only}
-
- Zhao XJ. The curative effect comparison of erythromycin‐benzoyl peroxide and Cuo Chuang Wang in treating acne vulgaris. Chinese Journal of Dermatovenereology 2001;15(4):288. [CENTRAL: CN‐00454983]
Zheng 2019 {published data only}
-
- Zheng Y, Yin S, Xia Y, Chen J, Ye C, Zeng Q, et al. Efficacy and safety of 2% supramolecular salicylic acid compared with 5% benzoyl peroxide/0.1% adapalene in the acne treatment: a randomized, split‐face, open‐label, single‐center study. Cutaneous & Ocular Toxicology 2019;38(1):48‐54. [CENTRAL: CN‐01741062] - PubMed
References to studies excluded from this review
2009‐011212‐37 {published data only}
-
- 2009‐011212‐37/PL. Anti P acnes activity of Epiduo® Gel compared to benzoyl peroxide 2.5% gel in the treatment of subjects with acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/trial/2009‐011212‐37/PL (first received 17 August 2009).
2009‐016240‐40 {published data only}
-
- 2009‐016240‐40/GR. Multicenter, randomized, double‐blind, comparative with the reference product clinical study to evaluate the efficacy and safety of the therapy with the combination of benzoyl peroxide‐erythromycin/verisfield, gel, (5+3)% w/w for the topical treatment of acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/trial/2009‐016240‐40/GR (first received 16 April 2010).
2010‐020796‐24 {published data only}
-
- 2010‐020796‐24/DE. A multi‐center, randomized, observer‐blind trial to compare the irritant potential of the two topical acne formulations Acanya® Gel and Epiduo® Gel on acneic skin in a split‐face assessment during a 14‐day treatment period. www.clinicaltrialsregister.eu/ctr‐search/trial/2010‐020796‐24/DE (first received 10 August 2010).
2013‐001753‐26 {published data only}
-
- 2013‐001753‐26/FR. Clinical and biophysics evaluation of the cutaneous modifications following the local use of a lotion containing 0,1 % of trétinoïne. www.clinicaltrialsregister.eu/ctr‐search/trial/2013‐001753‐26/FR (first received 28 September 2015).
2016‐000616‐15 {published data only}
-
- 2016‐000616‐15/PL. An open label evaluation of the adrenal suppression potential and trough plasma concentrations of cortexolone 17α‐propionate (CB‐03‐01) cream applied every 12 hours for two weeks in subjects 9 to <12 years of age with acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/trial/2016‐000616‐15/PL (first received 7 April 2016).
2017‐002975‐25 {published data only}
-
- 2017‐002975‐25/DK. Multimodal optical imaging for pretreatment evaluation for cutaneous microparticle delivery. www.clinicaltrialsregister.eu/ctr‐search/trial/2017‐002975‐25/DK (first received 20 September 2017).
2017‐003105‐18 {published data only}
-
- 2017‐003105‐18/NL. A randomized, placebo‐controlled, evaluator‐blinded study to assess the anti‐inflammatory effects of topical erythromycin and clindamycin in patients with inflammatory facial acne. www.clinicaltrialsregister.eu/ctr‐search/trial/2017‐003105‐18/NL (first received 12 December 2017).
ACTRN12617000498392 {published data only}
-
- ACTRN12617000498392. A single‐centre open‐label pharmacology study of topically applied MTC896 gel in healthy volunteers. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617000498392 (first received 6 April 2017).
ACTRN12617000642381 {published data only}
-
- ACTRN12617000642381. An open‐label study to evaluate the safety and tolerability of BTX1503 solution in healthy volunteers. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617000642381 (first received 3 May 2017).
ACTRN12617001127392 {published data only}
-
- ACTRN12617001127392. An open‐label study to evaluate the safety and tolerability of BTX 1503 solution in patients with acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12617001127392 (first received 1 August 2017).
Andres 2008 {published data only}
-
- Andres P, Pernin C, Poncet M. Adapalene‐benzoyl peroxide once‐daily, fixed‐dose combination gel for the treatment of acne vulgaris: a randomized, bilateral (split‐face), dose‐assessment study of cutaneous tolerability in healthy participants. Cutis; Cutaneous Medicine for the Practitioner 2008;81(3):278‐84. [CENTRAL: CN‐00639255; MEDLINE: ] - PubMed
Bhatia 2015 {published data only}
Bouloc 2017 {published data only}
-
- Bouloc A, Roo E, Imko‐Walczuk B, Moga A, Chadoutaud B, Dreno B. A skincare combined with combination of adapalene and benzoyl peroxide provides a significant adjunctive efficacy and local tolerance benefit in adult women with mild acne. Journal of the European Academy of Dermatology and Venereology 2017;31(10):1727‐31. [CENTRAL: CN‐01395998; MEDLINE: ] - PMC - PubMed
Bourdes 2015a {published data only}
-
- Bourdes V, Reynier P, Rivier M, Petit L, Tan J, Dreno B, et al. Adapalene/benzoyl peroxide fixed‐dose combination for the prevention and treatment of atrophic acne scars. British Journal of Dermatology 2015;173:63. [CENTRAL: CN‐01108072]
Bourdes 2015b {published data only}
-
- Bourdes V, Faure C, Petit L, Bettoli V, Bissonnette R, Dreno B, et al. Modeling of natural history of primary acne lesions and evolution to acne scars. Journal of the American Academy of Dermatology 2015;72(5 Suppl 1):AB8. [CENTRAL: CN‐01088982; DOI: 10.1016/j.jaad.2015.02.041] - DOI
Bucknall 1977 {published data only}
-
- Bucknall JH, Murdoch PN. Comparison of tretinoin solution and benzoyl peroxide lotion in the treatment of acne vulgaris. Current Medical Research & Opinion 1977;5(3):266‐8. [CENTRAL: CN‐00083593; MEDLINE: ] - PubMed
Burkhart 2007 {published data only}
-
- Burkhart CG, Burkhart CN. Treatment of acne vulgaris without antibiotics: tertiary amine‐benzoyl peroxide combination vs. benzoyl peroxide alone (Proactiv Solution). International Journal of Dermatology 2007;46(1):89‐93. [MEDLINE: ] - PubMed
Callender 2012 {published data only}
-
- Callender VD. Fitzpatrick skin types and clindamycin phosphate 1.2%/benzoyl peroxide gel: efficacy and tolerability of treatment in moderate to severe acne. Journal of Drugs in Dermatology 2012;11(5):643‐8. [CENTRAL: CN‐00880493; MEDLINE: ] - PubMed
Caron 1997 {published data only}
-
- Caron D, Sorba V, Clucas A, Verschoore M. Skin tolerance of adapalene 0.1% gel in combination with other topical antiacne treatments. Journal of the American Academy of Dermatology 1997;36(6 Pt 2):S113‐5. [CENTRAL: CN‐00141061; MEDLINE: ] - PubMed
Cavicchini 1989 {published data only}
-
- Cavicchini S, Caputo R. National and international experiences with azelaic acid cream in the treatment of papulo‐pustular acne. Giornale Italiano di Dermatologia e Venereologia 1989;124(10):465‐70. [MEDLINE: ] - PubMed
Choudhury 2011 {published data only}
-
- Choudhury S, Chatterjee S, Sarkar DK, Dutta RNCP. Efficacy and safety of topical nadifloxacin and benzoyl peroxide versus clindamycin and benzoyl peroxide in acne vulgaris: a randomized controlled trial. Indian Journal of Pharmacology 2011;43(6):628‐31. [CENTRAL: CN‐01003272; MEDLINE: ] - PMC - PubMed
Coret 2006 {published data only}
-
- Coret C, Chantalat J, Miller D, Kurtz E. Fast‐acting treatment of mild to moderate acne lesions by a novel 2% salicylic acid microgel complex. Abstract P115. American Academy of Dermatology 64th Annual Meeting, March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB18. [CENTRAL: CN‐00602686]
CTRI/2017/07/009004 {published data only}
-
- CTRI/2017/07/009004. Clinical evaluation of Unani formulations in the management of Busoor Labaniyah (Acne vulgaris). apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/07/009004 (first received 10 July 2017).
CTRI/2017/08/009299 {published data only}
-
- CTRI/2017/08/009299. Cinical evaluation of Vatapallava (tender leaves of Ficus benghalensis Linn) in Mukhadushika (acne vulgaris). apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/08/009299 (first received 8 August 2017).
CTRI/2017/09/009855 {published data only}
-
- CTRI/2017/09/009855. A phase 2, multicenter, randomized, double blind, comparative study to evaluate the reduction in incidence of scarring in acne vulgaris subjects treated with combination of benzoyl peroxide (2.5%/5%), zinc oxide and polysiloxanes compared to benzoyl peroxide (2.5%/5%). apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/09/009855 (first received 20 September 2017).
CTRI/2017/12/010963 {published data only}
-
- CTRI/2017/12/010963. Redefining the role of metformin in non‐hormonal acne: a single‐blind randomized placebo controlled interventional study comparing the efficacy of oral metformin and topical BPO vs. placebo and topical BPO; with initial 1 month of doxycycline, in the treatment of moderate‐severe facial acne. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/12/010963 (first received 22 December 2017).
Cunliffe 1981 {published data only}
-
- Cunliffe WJ, Holland KT. The effect of benzoyl peroxide on acne. Acta Dermato‐Venereologica 1981;61(3):267‐9. [CENTRAL: CN‐00025586] - PubMed
Degreef 1982 {published data only}
-
- Degreef H, Vanden Bussche G. Double‐blind evaluation of a miconazole‐benzoyl peroxide combination for the topical treatment of acne vulgaris. Dermatologica 1982;164(3):201‐8. [CENTRAL: CN‐00028058; MEDLINE: ] - PubMed
Del Rosso 2006b {published data only}
-
- Rosso J, Bikowski J, Desai A, Hawkes S. Management of truncal acne vulgaris: a double‐blind, randomized trial evaluating the clinical efficacy and tolerability of benzoyl peroxide 8% wash. Abstract P161. American Academy of Dermatology 64th Annual Meeting, March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB29. [CENTRAL: CN‐00602670]
Del Rosso 2009b {published data only}
Del Rosso 2010 {published data only}
-
- Rosso J. The aesthetic characteristics of a novel 6% benzoyl peroxide foaming cloth may improve adherence to acne therapy. Journal of the American Academy of Dermatology 2010;62(3 Suppl):AB15. [CENTRAL: CN‐00843673]
Del Rosso 2016 {published data only}
-
- Rosso JQ. When do efficacy outcomes in clinical trials correlate with clinical relevance? analysis of clindamycin phosphate 1.2%‐benzoyl peroxide 3.75% gel in moderate to severe acne vulgaris. Cutis; Cutaneous Medicine for the Practitioner 2016;98(1):21‐5. [MEDLINE: ] - PubMed
de Souza Sittart 2015 {published data only}
-
- Souza Sittart JA, Costa A, Mulinari‐Brenner F, Follador I, Azulay‐Abulafia L, Castro LCM. Multicenter study for efficacy and safety evaluation of a fixed dose combination gel with adapalene 0.1% and benzoyl peroxide 2.5% (Epiduo) for the treatment of acne vulgaris in Brazilian population. Anais Brasileiros de Dermatologia 2015;90(6 Suppl 1):S01‐16. [CENTRAL: CN‐01286635; MEDLINE: ] - PMC - PubMed
Dhawan 2009 {published data only}
-
- Dhawan SS. Comparison of 2 clindamycin 1%‐benzoyl peroxide 5% topical gels used once daily in the management of acne vulgaris. Cutis; Cutaneous Medicine for the Practitioner 2009;83(5):265‐72. [CENTRAL: CN‐00698409; MEDLINE: ] - PubMed
Dosik 2006 {published data only}
-
- Dosik JS, Gilbert RD, Arsonnaud S. Cumulative irritancy comparison of topical retinoid and antimicrobial combination therapies. Skinmed 2006;5(5):219‐23. [CENTRAL: CN‐00572027; MEDLINE: ] - PubMed
Dosik 2008 {published data only}
-
- Dosik JS, Vamvakias G. Comparative irritation potential of two combination acne products: a randomized controlled, subject‐ and evaluator‐blind, comparative study in healthy volunteers. American Journal of Clinical Dermatology 2008;9(5):313‐7. [CENTRAL: CN‐00668403; MEDLINE: ] - PubMed
Draelos 2006 {published data only}
-
- Draelos Z, Callender V, Dhawan S. Tolerability and preference of two benzoyl peroxide/clindamycin gels in combination with tretinoin 0.025% cream in adult female acne patients. Abstract P176. American Academy of Dermatology 64th Annual Meeting, March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB33. [CENTRAL: CN‐00602662]
Draelos 2012 {published data only}
-
- Draelos ZD, Shalita AR, Thiboutot D, Oresajo C, Yatskayer M, Raab S. A multicenter, double‐blind study to evaluate the efficacy and safety of 2 treatments in participants with mild to moderate acne vulgaris. Cutis; Cutaneous Medicine for the Practitioner 2012;89(6):287‐93. [CENTRAL: CN‐00831065; MEDLINE: ] - PubMed
Draelos 2015 {published data only}
-
- Draelos ZD. Assessing the value of botanical anti‐inflammatory agents in an OTC acne treatment regimen. Journal of Drugs in Dermatology 2015;14(12):1418‐21. [CENTRAL: CN‐01200536; MEDLINE: ] - PubMed
DRKS00010222 {published data only}
-
- DRKS00010222. Application of a cosmetic product on facial acne skin. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00010222 (first received 17 May 2016).
Eichenfield 2009 {published data only}
-
- Eichenfield LF, Thiboutot D, Shalita A, Swinyer L, Tanghetti E, Tschen E, et al. A three‐step acne system containing solubilized benzoyl peroxide versus benzoyl peroxide/clindamycin in pediatric patients with acne. Journal of Clinical and Aesthetic Dermatology 2009;2(11):21‐6. [CENTRAL: CN‐00802895; MEDLINE: ] - PMC - PubMed
Eichenfield 2010b {published data only}
-
- Eichenfield L, Shalita A, Thiboutot D, Swinyer L. A 3‐step acne system containing solubilized benzoyl peroxide versus benzoyl peroxide‐clindamycin in pediatric patients with acne (Poster 705). 68th Annual Meeting of the American Academy of Dermatology, AAD Miami, FL United States. Journal of the American Academy of Dermatology 2010;62(3 Suppl):AB14. [CENTRAL: CN‐00843674]
Ergin 1999 {published data only}
-
- Ergin S, et al. Benzoyl peroxide alone and with topical erythromycin against Propionibacterium acnes in acne vulgaris. Abstract P‐004. 8th Congress of the European Academy of Dermatology and Venereology. Amsterdam, The Netherlands, 29 Sept‐3 October 1999. Journal of the European Academy of Dermatology and Venereology 1999;12(Suppl 2):S142. [CENTRAL: CN‐00478524]
Ergin 2001 {published data only}
-
- Ergin S, Ergin C, Baysal V, Yayli G. An acne study focused on erythromycin: benzoyl peroxide alone or with topical erythromycin against Propionibacterium acnes in acne vulgaris. Gazi Medical Journal 2001;12(2):59‐62. [CENTRAL: CN‐00386806]
Fanta 1984 {published data only}
-
- Fanta D, Scholz N. Miconazole‐benzoyl peroxide: a new combination to extend the topical therapy of acne. Zeitschrift fur Hautkrankheiten 1984;59(13):873‐81. [CENTRAL: CN‐00035219] - PubMed
Fernandez‐Obregon 2003 {published data only}
-
- Fernandez‐Obregon A, Davis MW. The BEST study: evaluating efficacy by selected demographic subsets. Cutis; Cutaneous Medicine for the Practitioner 2003;71(2 Suppl):18‐26. [MEDLINE: ] - PubMed
Fluckiger 1988 {published data only}
-
- Fluckiger R, Furrer HJ, Rufli T. Efficacy and tolerance of a miconazole‐benzoyl peroxide cream combination versus a benzoyl peroxide gel in the topical treatment of acne vulgaris. Dermatologica 1988;177(2):109‐14. [CENTRAL: CN‐00055898; MEDLINE: ] - PubMed
Ghosh 2018 {published data only}
-
- CTRI/2017/08/009280. Efficacy and safety of nadifloxacin and benzoyl peroxide versus adapalene and benzoyl peroxide in acne vulgaris: a randomized open label phase IV clinical trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/08/009280 (first received 7 August 2017).
-
- Ghosh A, Das K. Efficacy and safety of nadifloxacin and benzoyl peroxide versus adapalene and benzoyl peroxide in acne vulgaris: a randomized open‐label Phase IV clinical trial. Journal of Pharmacology & Pharmacotherapeutics 2018;9(1):27‐31.
Gonzalez 2012 {published data only}
-
- Gonzalez P, Vila R, Cirigliano M. The tolerability profile of clindamycin 1%/benzoyl peroxide 5% gel vs. adapalene 0.1%/benzoyl peroxide 2.5% gel for facial acne: results of a randomized, single‐blind, split‐face study. Journal of Cosmetic Dermatology 2012;11(4):251‐60. [CENTRAL: CN‐00878198; MEDLINE: ] - PubMed
Green 2008 {published data only}
-
- Green LJ, Rosso JQ. Efficacy and tolerability of a three‐step acne system containing a solubilized benzoyl peroxide lotion versus a benzoyl peroxide/clindamycin combination product: an investigator‐blind, randomized, parallel‐group study. Journal of Clinical and Aesthetic Dermatology 2008;1(3):16‐20. [MEDLINE: ] - PMC - PubMed
Green 2012 {published data only}
-
- Green L, Cirigliano M, Gwazdauskas JA, Gonzalez P. The tolerability profile of clindamycin 1%/benzoyl peroxide 5% gel vs. adapalene 0.1%/benzoyl peroxide 2.5% gel for facial acne: results of two randomized, single‐blind, split‐face studies. Journal of Clinical and Aesthetic Dermatology 2012;5(5):16‐24. [CENTRAL: CN‐00904062; MEDLINE: ] - PMC - PubMed
Green 2013 {published data only}
-
- Green L, Kircik LH, Gwazdauskas J. Randomized, controlled, evaluator‐blinded studies conducted to compare the efficacy and tolerability of 3 over‐the‐counter acne regimens in subjects with mild or moderate acne. Journal of Drugs in Dermatology 2013;12(2):180‐5. [CENTRAL: CN‐00877485; MEDLINE: ] - PubMed
Grove 2013 {published data only}
-
- Grove G, Zerweck C, Gwazdauskas J. Tolerability and irritation potential of four topical acne regimens in healthy subjects. Journal of Drugs in Dermatology 2013;12(6):644‐9. [CENTRAL: CN‐01122622; MEDLINE: ] - PubMed
IRCT2016051727947N1 {published data only}
-
- IRCT2016051727947N1. Comparing the effect of benzoyl peroxide 5% and IPL and benzoyl peroxide 5%‐alone in mild‐to‐moderate acne. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016051727947N1 (first received 20 June 2016).
ISRCTN21526350 {published data only}
-
- ISRCTN21526350. Identification of the most cost effective, microbiologically safe antimicrobial treatments for acne. apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN21526350 (first received 25 April 2003).
ISRCTN38383374 {published data only}
-
- ISRCTN38383374. Efficacy and local tolerability of different spray products in the treatment of mild to moderate acne of the back and chest: a controlled, three‐arm, assessor‐blinded prospective trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN38383374 (first received 5 May 2016).
Jain 1998 {published data only}
-
- Jain VK, Chopra KL, Dayal S. Comparative evaluation of topical benzoyl peroxide, metronidazole and benzoyl peroxide ‐ clindamycin combination in treatment of acne vulgaris. Indian Journal of Dermatology, Venerology and Leprology 1998;64(2):71‐4. [CENTRAL: CN‐01095038; MEDLINE: ] - PubMed
Jeanmougin 1987 {published data only}
-
- Jeanmougin M. Minocycline and benzoyl peroxide in treatment of acne. Results of a multicenter trial with 256 patients [Minocycline et peroxyde de benzoyle dans le traitement de l'acne resultats s'une etude multicentrique sur 256 patients]. Comptes Rendus de Therapeutique et de Pharmacologie Clinique 1987;5(50):3, 5, 7‐9, 11. [CENTRAL: CN‐01599518]
JPRN‐UMIN000025358 {published data only}
-
- JPRN‐UMIN000025358. Quantitative determination of nadifloxacin in follicles of acne patients treated by topical nadifloxacin with or without adapalene and benzoyl peroxide. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000025358 (first received 21 December 2016).
KCT0002259 {published data only}
-
- KCT0002259. Effects of herbal medicine for dysmenorrhea treatment on accompanied acne vulgaris: double‐blind, randomized, parallel‐group, multi‐center trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=KCT0002259 (first received 10 March 2017).
Kellett 2006 {published data only}
-
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient preferences for topical antibiotic treatment for acne. A randomised controlled trial. British Journal of Dermatology 2006;154(3):524‐32. [CENTRAL: CN‐00562346; MEDLINE: ] - PubMed
Kircik 2006 {published data only}
-
- Kircik L. Study results of the evaluation of contamination measurements of benzoyl peroxide 5%/clindamycin 1% topical gel jar containing dimethicone and glycerin compared with topical gel tube. Abstract P593. American Academy of Dermatology 64th Annual Meeting, March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB74. [CENTRAL: CN‐00602584]
Kircik 2007 {published data only}
-
- Kircik L. Community‐based trial results of combination clindamycin 1%‐benzoyl peroxide 5% topical gel plus tretinoin microsphere Gel 0.04% or 0.1% or adapalene gel 0.1% in the treatment of moderate to severe acne. Cutis; Cutaneous Medicine for the Practitioner 2007;80(1 Suppl):10‐4. [CENTRAL: CN‐00611938] - PubMed
Kircik 2009a {published data only}
-
- Kircik L, Green L, Thiboutot D, Tanghetti E, Wilson D, Dhawan S, et al. Comparing a novel solubilized benzoyl peroxide gel with benzoyl peroxide/clindamycin: final data from a multicenter, investigator‐blind, randomized study. Journal of Drugs in Dermatology 2009;8(9):812‐8. [CENTRAL: CN‐00722784; MEDLINE: ] - PubMed
Kircik 2009b {published data only}
-
- Kircik LH. Comparative efficacy and safety results of two topical combination acne regimens. Journal of Drugs in Dermatology 2009;8(7):624‐30. [CENTRAL: CN‐00702099; MEDLINE: ] - PubMed
Leyden 1997a {published data only}
-
- Leyden JJ, Gans EH. Evaluation of the antimicrobial effects in vivo of Triaz (R) Gel (benzoyl peroxide special gel), Cleocin‐T (R) Lotion (clindamycin phosphate lotion), and Azelex (R) Cream (azelaic acid cream) in humans. Journal of Dermatological Treatment 1997;8(Suppl 2):S7‐S10. [CENTRAL: CN‐00191892]
Leyden 1997b {published data only}
-
- Leyden JJ, Gans EH. The human in vivo antimicrobial effects of dual acne therapy: Oral Dynacin (minocycline HCl) plus topical Triaz (benzoyl peroxide special gel). Journal of Dermatological Treatment 1997;8:S3‐6.
Leyden 2010 {published data only}
-
- Leyden JJ, Nighland M, Rossi AB, Ramaswamy R. Irritation potential of tretinoin gel microsphere pump versus adapalene plus benzoyl peroxide gel. Journal of Drugs in Dermatology 2010;9(8):998‐1003. [CENTRAL: CN‐00749513; MEDLINE: ] - PubMed
Mareledwane 2006 {published data only}
-
- Mareledwane NG. A randomized, open‐label, comparative study of oral doxycycline 100 mg vs. 5% topical benzoyl peroxide in the treatment of mild to moderate acne vulgaris. International Journal of Dermatology 2006;45(12):1438‐9. [CENTRAL: CN‐00574632; MEDLINE: ] - PubMed
Mesquita 1989 {published data only}
-
- Mesquita Guimaraes J, Ramos S, Tavares MR, Carvalho MR. A double‐blind clinical trial with a lotion containing 5% benzoyl peroxide and 2% miconazole in patients with acne vulgaris. Clinical and Experimental Dermatology 1989;14(5):357‐60. [CENTRAL: CN‐00065083; MEDLINE: ] - PubMed
Miller 2005 {published data only}
-
- Miller D, Smith G, Kurtz E S. Improvement in mild to moderate inflammatory acne lesions with a novel salicylic acid topical acne treatment. Abstract P01.98. The 14th Congress of the European Academy of Dermatology and Venereology, London, UK, 12‐15 October 2005. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):25‐6. [CENTRAL: CN‐00602638]
Miller 2006a {published data only}
-
- Miller T, Ramirez J. Benzoyl peroxide enhanced Propionibacterium acnes reduction from a novel topical formulation. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB24. [CENTRAL: CN‐00602649]
Miller 2006b {published data only}
-
- Miller T, Ramirez J, Goldner S. Improved Propionibacterium acnes reduction from a novel benzoyl peroxide formulation. Abstract P158. American Academy of Dermatology 64th Annual Meeting, March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB28. [CENTRAL: CN‐00602614]
NCT00441415 {published data only}
-
- NCT00441415. Efficacy and safety of fixed combination adapalene 0.1%/benzoyl peroxide 2.5% gel compared to clindamycin 0.1%/benzoyl peroxide 5% gel in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT00441415 (first received 27 February 2007).
NCT00687908 {published data only}
-
- NCT00687908. Adapalene‐benzoyl peroxide (BPO) gel in the treatment of acne vulgaris as a 6‐month maintenance (ACCESS II). www.clinicaltrials.gov/ct2/show/NCT00687908 (first received 28 May 2008).
NCT00757523 {published data only}
-
- NCT00757523. Evaluation of the effectiveness, safety, and tolerability of Duac Akne Gel and Epiduo Gel in the treatment of facial acne vulgaris. clinicaltrials.gov/ct2/show/NCT00757523 (first received 21 September 2008).
NCT00919191 {published data only}
-
- NCT00919191. Evaluation of irritation by two facial gels applied to opposite sides of the face. www.clinicaltrials.gov/ct2/show/NCT00919191 (first received 10 June 2009).
NCT00926367 {published data only}
-
- NCT00926367. Two‐week study to compare the tolerance and irritation potential of two combination topical gel acne medications. clinicaltrials.gov/ct2/show/NCT00926367 (first received 21 June 2009).
NCT00952523 {published data only}
-
- NCT00952523. Evaluation of irritation that potentially could be caused by two facial gels applied to opposite sides of the face. www.clinicaltrials.gov/ct2/show/NCT00952523 (first received 4 August 2009).
NCT00964223 {published data only}
-
- NCT00964223. A study to evaluate tolerability of two topical drug products in the treatment of facial acne. www.clinicaltrials.gov/ct2/show/NCT00964223 (first received 20 August 2009).
NCT00964366 {published data only}
-
- NCT00964366. Study to determine and compare the tolerance and irritation potential of topical acne medications. clinicaltrials.gov/ct2/show/NCT00964366 (first received 20 August 2009).
NCT01015638 {published data only}
-
- NCT01015638. Compare the tolerance of clindamycin 1%/benzoyl peroxide (BPO) 5% gel to clindamycin 1.2%/BPO 2.5% topical medications. clinicaltrials.gov/ct2/show/NCT01015638 (first received 17 November 2009).
NCT01188538 {published data only}
-
- NCT01188538. Anti Propionibacterium (P) acnes activity of Epiduo® gel compared to benzoyl peroxide (BPO) 2.5% gel. clinicaltrials.gov/ct2/show/NCT01188538 (first received 24 August 2010).
NCT01613924 {published data only}
-
- NCT01613924. Efficacy of handheld acne heat device. www.clinicaltrials.gov/ct2/show/NCT01613924 (first received 30 May 2012).
NCT01706250 {published data only}
-
- NCT01706250. U0289‐401: eight week, split‐face study to determine and compare the efficacy and tolerability of MAXCLARITY™ II to PROACTIV™. www.clinicaltrials.gov/ct2/show/NCT01706250 (first received 11 October 2012).
NCT01818167 {published data only}
-
- NCT01818167. An investigation Into the efficacy of povidone topical cream as compared to 10% benzoyl peroxide wash for the treatment of hidradenitis suppurativa. clinicaltrials.gov/ct2/show/NCT01818167 (first received 20 March 2013).
NCT02052752 {published data only}
-
- NCT02052752. A five day clinical study to examine the effects of a benzoyl peroxide treatment on facial acne lesions. clinicaltrials.gov/ct2/show/NCT02052752 (first received 30 January 2014).
NCT02524665 {published data only}
-
- NCT02524665. 8 week study to evaluate and compare the efficacy and tolerability of MAXCLARITY II and MURAD to treat acne. clinicaltrials.gov/ct2/show/NCT02524665 (first received 13 August 2015).
NCT02589405 {published data only}
-
- NCT02589405. Benzac 5% gel in combination with cosmetic products in acne vulgaris (Benzac). clinicaltrials.gov/ct2/show/NCT02589405 (first received 23 October 2015).
NCT02698436 {published data only}
-
- NCT02698436. A study to evaluate the effectiveness and tolerance of two acne treatments on subjects with mild to moderate acne (TIGER). clinicaltrials.gov/ct2/show/NCT02698436 (first received 26 February 2016).
NCT02899000 {published data only}
-
- NCT02899000. A treatment for severe inflammatory acne subjects. clinicaltrials.gov/ct2/show/NCT02899000 (first received 8 September 2016).
NCT02932267 {published data only}
-
- NCT02932267. Subject reported outcomes with use of adapalene 0.3% ‐ benzoyl peroxide (BPO) 2.5% in dark skin acne (EDeN). clinicaltrials.gov/ct2/show/NCT02932267 (first received 11 October 2016).
NCT03057821 {published data only}
-
- NCT03057821. Propionibacterium acnes in shoulder arthroplasty. clinicaltrials.gov/ct2/show/NCT03057821 (first received 14 February 2017).
NCT03122457 {published data only}
-
- NCT03122457. Perimenstrual acne with clindamycin phosphate and benzoyl peroxide. clinicaltrials.gov/ct2/show/NCT03122457 (first received 17 April 2017).
NCT03128723 {published data only}
-
- NCT03128723. A study to evaluate the tolerance of an acne treatment in sensitive skin subjects with mild to moderate acne vulgaris. clinicaltrials.gov/ct2/show/NCT03128723 (first received 20 April 2017).
NCT03257202 {published data only}
-
- NCT03257202. Topical treatment and prevalence of P. acnes. clinicaltrials.gov/ct2/show/NCT03257202 (first received 1 August 2017).
NCT03334682 {published data only}
-
- NCT03334682. Randomized double‐blind study on the benefit of spironolactone for treating acne of adult woman (FASCE). clinicaltrials.gov/ct2/show/NCT03334682 (first received 30 October 2017).
Ozdemir 2005 {published data only}
-
- Ozdemir M, Bahar H, Erakkaya H, Mamal M, Kotogyan A, Yucel A, et al. Effect of 10% benzoyl peroxide/10% glycolic acid versus 5% benzoyl peroxide/3% erythromycin in clinical efficacy and reduction of Propionibacterium acnes in acne vulgaris. Abstract P01.14. 14th Congress of the European Academy of Dermatology and Venereology, London, UK, 12‐15 October 2005. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):4. [CENTRAL: CN‐00602563]
Ozolins 2002a {published data only}
-
- Ozolins M, Eady EA, Avery A, Cunliffe WJ, Li Wan Po A, O'Neill C, et al. A cost‐effective rationale for the selection of antimicrobial therapy in acne: a randomized controlled trial. British Journal of Dermatology 2002;147(Suppl 62):13. [CENTRAL: CN‐00406984]
Ozolins 2002b {published data only}
-
- Ozolins M, Eady EA, Avery A, Cunliffe WJ, Li Wan Po A, O'Neill C, et al. A cost‐effectiveness rationale for the selection of antimicrobial therapy in acne: a randomized controlled trial (Abstract RF‐2). 82nd BAD Annual Meeting, 9‐12 July 2002. British Journal of Dermatology 2002;147:13. [CENTRAL: CN‐00406984]
Ozolins 2004 {published data only}
-
- Ozolins M, Eady E, Avery AJ, Cunliffe WJ, Po AL, O'Neill C, et al. Comparison of five antimicrobial regimens for treatment of mild to moderate inflammatory facial acne vulgaris in the community: randomised controlled trial. Lancet 2004;364(9452):2188‐95. [CENTRAL: CN‐00503549; MEDLINE: ] - PubMed
Ozolins 2005 {published data only}
-
- Ozolins M, Eady EA, Avery A, Cunliffe WJ, O'Neill C, Simpson NB, et al. Randomised controlled multiple treatment comparison to provide a cost‐effectiveness rationale for the selection of antimicrobial therapy in acne. Health Technology Assessment (Winchester, England) 2005;9(1):iii‐212. [CENTRAL: CN‐00514416; MEDLINE: ] - PubMed
Pariser 2010 {published data only}
-
- Pariser D, Bucko A, Fried R, Jarratt MT, Kempers S, Kircik L, et al. Tretinoin gel microsphere pump 0.04% plus 5% benzoyl peroxide wash for treatment of acne vulgaris: morning/morning regimen is as effective and safe as morning/evening regimen. Journal of Drugs in Dermatology 2010;9(7):805‐13. [CENTRAL: CN‐00752706; MEDLINE: ] - PubMed
Patel 2001 {published data only}
Poulin 2010 {published data only}
-
- Poulin Y, Sanchez NP, Bucko A, Fowler J, Jarratt M, Kempers S, et al. A 6‐month maintenance therapy with adapalene‐benzoyl peroxide gel prevents relapse and continuously improves efficacy among patients with severe acne vulgaris: results of a randomized controlled trial. British Journal of Dermatology 2010;164(6):1376‐82. [CENTRAL: CN‐00812175; MEDLINE: ] - PubMed
Richter 2016 {published data only}
-
- Richter C, Trojahn C, Hillmann K, Dobos G, Stroux A, Kottner J, et al. Reduction of inflammatory and noninflammatory lesions with topical tyrothricin 0.1% in the treatment of mild to severe acne papulopustulosa: a randomized controlled clinical trial. Skin Pharmacology and Physiology 2016;29(1):1‐8. [CENTRAL: CN‐01138615; MEDLINE: ] - PubMed
Rodriguez 2003 {published data only}
-
- Rodriguez D, Davis MW. The BEST study: results according to prior treatment. Cutis; Cutaneous Medicine for the Practitioner 2003;71(2 Suppl):27‐34. [MEDLINE: ] - PubMed
Rueda 2014 {published data only}
Schlesinger 2010 {published data only}
-
- Schlesinger T, Mills OH, Repaire R. An open community‐based trial with combination topical (1% salicylic acid and 4% benzoyl peroxide/2% salicylic acid and 8% benzoyl peroxide) and 5% tocopherol therapy for acne vulgaris. P708. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB15. [https://www.jaad.org/article/S0190‐9622(09)01573‐4/pdf]
Schutte 1982 {published data only}
-
- Schutte H, Cunliffe WJ, Forster RA. The short‐term effects of benzoyl peroxide lotion on the resolution of inflamed acne lesions. British Journal of Dermatology 1982;106(1):91‐4. [CENTRAL: CN‐00568905; MEDLINE: ] - PubMed
Shemer 2009 {published data only}
-
- Shemer A, Mavor D, Drori E, Barsimantov H, Toledano O. Time‐release silica encapsulation in antiacne treatments (Poster P718). 68th Annual Meeting of the American Academy of Dermatology, AAD Miami, FL United States. Journal of the American Academy of Dermatology 2009;62(3 Suppl):AB18. [CENTRAL: CN‐00843856]
Tanghetti 2008 {published data only}
-
- Tanghetti E, Kircik L, Wilson D, Dhawan S. Solubilized benzoyl peroxide versus benzoyl peroxide/clindamycin in the treatment of moderate acne. Journal of Drugs in Dermatology 2008;7(6):534‐8. [CENTRAL: CN‐00640327; MEDLINE: ] - PubMed
TCTR20160216002 {published data only}
-
- TCTR20160216002. The study of the effectiveness of silver nanoparticle for the treatment of acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20160216002 (first received 15 February 2016).
TCTR20170603001 {published data only}
-
- TCTR20170603001. A comparison of the efficacy and safety of 0.1% adapalene gel and 0.025% tretinoin cream in the treatment of childhood acanthosis nigricans. apps.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20170603001 (first received 3 June 2017).
TCTR20171104001 {published data only}
-
- TCTR20171104001. A randomized, controlled, split‐face clinical trial comparing combination of ProACNE SOLUTION ACTIVE CLEAR with 2.5% benzoyl peroxide versus 2.5% benzoyl peroxide with placebo in the treatment of mild to moderate degree of acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20171104001 (first received 3 November 2017).
Touitou 2008 {published data only}
-
- Touitou E, Godin B, Shumilov M, Bishouty N, Ainbinder D, Shouval R, et al. Efficacy and tolerability of clindamycin phosphate and salicylic acid gel in the treatment of mild to moderate acne vulgaris. Journal of the European Academy of Dermatology and Venereology 2008;22(5):629‐31. [CENTRAL: CN‐00631273; MEDLINE: ] - PubMed
Veraldi 2016 {published data only}
-
- Veraldi S, Micali G, Berardesca E, Dall'Oglio F, Sinagra Jl, Guanziroli E. Results of a multicenter, randomized, controlled trial of a hydrogen peroxide‐based kit versus a benzoyl peroxide‐based kit in mild‐to‐moderate acne. Journal of Clinical and Aesthetic Dermatology 2016;9(10):50‐4. [CENTRAL: CN‐01246601; MEDLINE: ] - PMC - PubMed
Weiss 2003a {published data only}
-
- Weiss J, Shavin J, Davis MW. Overall results of the BEST study following treatment of patients with mild to moderate acne. Cutis; Cutaneous Medicine for the Practitioner 2003;71(2 Suppl):10‐7. [MEDLINE: ] - PubMed
Weiss 2003b {published data only}
-
- Weiss J, Shavin J, Davis MW. Improving patient satisfaction and acne severity in patients with mild to moderate acne: the BEST study. Cutis; Cutaneous Medicine for the Practitioner 2003;71(2 Suppl):3‐4. [MEDLINE: ] - PubMed
Weiss 2011 {published data only}
-
- Weiss JS, Bucko AD, Fowler JF, Jarratt MT, Kempers S. Effective 6‐month maintenance management of acne with adapalene benzoyl peroxide fixed‐dose combination. (Abstract P710). Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB15. [CENTRAL: CN‐00843982]
Wilhelm 2011 {published data only}
-
- Wilhelm KP, Wilhelm D, Neumeister C, Zsolt I, Schwantes U. Lack of irritative potential of nadifloxacin 1% when combined with other topical anti‐acne agents. Clinical & Experimental Dermatology 2012;37(2):112‐7. [CENTRAL: CN‐00841319; MEDLINE: ] - PubMed
Wilson 2007 {published data only}
-
- Wilson DC, Meadows KP. A comparison of a novel benzoyl peroxide system with a combination benzoyl peroxide and clindamycin product: a 2‐week split face study of effectiveness and tolerability. Abstract P146. American Academy of Dermatology 65th Annual Meeting, February 2‐6, 2007. Journal of the American Academy of Dermatology 2007;56(2):AB24. [CENTRAL: CN‐00615964]
Woodruff 2009 {published data only}
-
- Woodruff J, Wallo W. Evaluating two different clinical approaches for treating mild to moderate acne. P722. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB18. [DOI: 10.1016/j.jaad.2008.11.103] - DOI
Zhen 2006 {published data only}
-
- Zhen YX, Crosby M, Stoudemayer M, Kligman AM. The moisturizing effects of topical acne medications. Abstract P174. American Academy of Dermatology 64th Annual Meeting, March 3‐7, 2006. Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB32. [CENTRAL: CN‐00602606; DOI: 10.1016/j.jaad.2005.11.117] - DOI
Zouboulis 2009a {published data only}
-
- Zouboulis CC, Fischer TC, Wohlrab J, Barnard J, Alio AB. Study of the efficacy, tolerability, and safety of 2 fixed‐dose combination gels in the management of acne vulgaris. Cutis; Cutaneous Medicine for the Practitioner 2009;84(4):223‐9. [CENTRAL: CN‐00728202; MEDLINE: ] - PubMed
Zouboulis 2009b {published data only}
-
- Zouboulis CC, Alio A. Efficacy, safety, and tolerability of two fixed‐dose combination gels for the treatment of acne vulgaris: results of the "DUETTA" study. Australasian Journal of Dermatology 2009;50:A56‐7.
Zouboulis 2010 {published data only}
-
- Zouboulis C, Alio A. Efficacy, safety, and tolerability of two fixed‐dose combination gels for the treatment of acne vulgaris: results of the DUETTA study (Poster P717). 68th Annual Meeting of the American Academy of Dermatology, AAD Miami, FL, United States. Journal of the American Academy of Dermatology 2010;62(3 Suppl):AB17. [CENTRAL: CN‐00843994]
References to studies awaiting assessment
2004‐002272‐41 {published data only}
-
- 2004‐002272‐41. A multi‐centre, single‐blind, parallel group, clinical evaluation of the efficacy and safety of Duac gel (a gel containing clindamycin phosphate [equivalent to 1% clindamycin] and 5% benzoyl peroxide) and Differin gel (a gel containing 0.1% adapalene) in the topical treatment of mild to moderate acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/trial/2004‐002272‐41/GB (first received 28 June 2005).
2006‐004278‐28 {published data only}
-
- 2006‐004278‐28/IT. Efficacy and safety of a fixed combination adapalene 0.1%/benzoyl peroxide 2.5% gel compared to clindamycin 1%/benzoyl peroxide 5% gel in the treatment of acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/trial/2006‐004278‐28/IT (first received 27 January 2009).
2008‐002359‐26 {published data only}
-
- 2008‐002359‐26. A multi‐center, randomized, evaluator‐blind, parallel group study evaluation of the efficacy, safety, and tolerability of DUAC® Akne Gel and Epiduo® Gel in the topical treatment of facial acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/trial/2008‐002359‐26 (first received 26 June 2008).
2008‐006792‐68 {published data only}
-
- 2008‐006792‐68. Efficacy and safety comparison of adapalene 0.1%/benzoyl peroxide 2.5% gel associated with lymecycline 300mg capsules versus adapalene 0.1%/benzoyl peroxide 2.5% vehicle gel associated with lymecycline 300mg capsules in the treatment of moderate to severe acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/trial/2008‐006792‐68 (first received 12 May 2009).
2013‐001716‐30 {published data only}
-
- 2013‐001716‐30. Exploratory, controlled, randomized, observer‐blind intrainidividual clinical trial to evaluate the efficacy and the tolerability of topically applied 0.1% tyrothricin (Tyrosur® Gel) in patients with mild to severe facial papulopustular acne. www.clinicaltrialsregister.eu/ctr‐search/trial/2013‐001716‐30 (first received 13 August 2013).
2016‐000063‐16 {published data only}
-
- 2016‐000063‐16/DE. Efficacy and safety of CD5024 1% in acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/trial/2016‐000063‐16/DE (first received 4 March 2016).
Ahmadi 2014 {published data only}
-
- Ahmadi Ashtiani H, Hooshange Ehsani A, Brikbin B, Krimlou Z, Javadi M. Evaluation of the effectiveness of formulation containing Cynara scolymus (Artichoke) leaf extract on epidermal growth factor (EGF) in older women with acne skin. Journal der Deutschen Dermatologischen Gesellschaft (Journal of the German Society of Dermatology) 2014;12:20.
Anonymous 1985 {published data only}
-
- Anonymous. Multicenter trial for aqueous gels with 5% and 10% benzoyl peroxide in treatment of acne vulgaris [Etude multicentrique d'eclaran (5 et 10) dans le traitement de l'acne vulgaire. A propos de 140 cas]. Comptes Rendus de Therapeutique et de Pharmacologie Clinique 1985;3(31):11‐4. [CENTRAL: CN‐00365235]
Chiou 2012 {published data only}
-
- Chiou WL. Low intrinsic drug activity and dominant vehicle (placebo) effect in the topical treatment of acne vulgaris. International Journal of Clinical Pharmacology & Therapeutics 2012;50(6):434‐7. [CENTRAL: CN‐00969115; MEDLINE: ] - PubMed
Cunliffe 1978 {published data only}
-
- Cunliffe WJ, Dodman B, Ead R. Benzoyl peroxide in acne. Practitioner 1978;220(1317):479‐82. [MEDLINE: ] - PubMed
Cunliffe 1980 {published data only}
-
- Cunliffe WJ, Burke B, Dodman B. Chloramphenicol and benzoyl peroxide in acne. A double‐blind clinical study. Practitioner 1980;224(1347):952‐4. [CENTRAL: CN‐00024025; MEDLINE: ] - PubMed
Dahl 2012 {published data only}
-
- Dahl A, Oresajo C, Baumann L, Yatskayer M. A split‐face clinical trial to compare the safety and efficacy of two topical acne treatments in subjects with mild to moderate acne vulgaris. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB22. [DOI: 10.1016/j.jaad.2011.11.101] - DOI
Danto 1966 {published data only}
-
- Danto JL, Maddin WS, Stewart WD, Nelson AJ. A controlled trial of benzoyl peroxide and precipitated sulfur cream in acne vulgaris. Applied Therapeutics 1966;8(7):624‐5. [CENTRAL: CN‐00000721; MEDLINE: ] - PubMed
Fagundes 2003 {published data only}
-
- Fagundes DS, Fraser JM, Klauda HC. New therapy update ‐ A unique combination formulation in the treatment of inflammatory acne. Cutis; Cutaneous Medicine for the Practitioner 2003;72(1 Suppl):16‐9. [CENTRAL: CN‐00450281; MEDLINE: ] - PubMed
IRCT20181229042165N1 {published data only}
-
- IRCT20181229042165N1. Effectiveness of adapalene 0.1% with intense pulsed light versus benzoyl peroxide 5% with intense pulsed light in the treatment of acne vulgaris: a comparative study. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20181229042165N1 (first received 8 January 2019).
Lassus 1981 {published data only}
-
- Lassus A. Local treatment of acne. A clinical study and evaluation of the effect of different concentrations of benzoyl peroxide gel. Current Medical Research & Opinion 1981;7(6):370‐3. [CENTRAL: CN‐00025227; MEDLINE: ] - PubMed
Leyden 2002 {published data only}
-
- Leyden JJ. Clindamycin 1%/benzoyl peroxide 5% is more effective than clindamycin alone in reducing Propionibacterium acnes (Abstract P1‐13). 11th Congress of the European Academy of Dermatology & Venereology, Prague, October 2‐6, 2002. Journal of the European Academy of Dermatology and Venereology 2002;16(Suppl 1):117. [CENTRAL: CN‐00416128]
Mallol 1984 {published data only}
-
- Mallol Miron J, Hurtado Manzano C, Parra Ortiz I. Evaluation of the efficacy of benzoyl peroxide at high concentrations. Therapeutic and microbiological study. Medicina Clinica 1984;83(17):705‐7. [CENTRAL: CN‐00268406; MEDLINE: ] - PubMed
NCT00160394 {published data only}
-
- Donald AE, Atkinson GE, Langner A, Chu A, Clayton T. Efficacy and safety of Duac Gel (a gel containing 1% clindamycin and 5% benzoyl peroxide) compared with Differin Gel (a gel containing 0.1% adapalene) in the topical treatment of mild to moderate acne vulgaris. Abstract P01.139. 14th Congress of the European Academy of Dermatology and Venereology, London, UK, 12‐15 October 2005. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):36. [CENTRAL: CN‐00602579]
-
- NCT00160394. Comparison of Duac® gel and Differin® gel in mild to moderate acne vulgaris. clinicaltrials.gov/ct2/show/NCT00160394 (first received 12 September 2005).
NCT00624676 {published data only}
-
- NCT00624676. Efficacy and tolerance of a derivative of salicylic acid and 5% benzoyl peroxide in facial acne vulgaris. clinicaltrials.gov/ct2/show/NCT00624676 (first received 27 February 2008).
NCT00663286 {published data only}
-
- NCT00663286. A clinical trial evaluating the safety and efficacy of IDP‐110 in patients with acne vulgaris. clinicaltrials.gov/ct2/show/NCT00663286 (first received 22 April 2008).
-
- NCT00664248. A clinical study evaluating the safety and efficacy of IDP‐110 in patients with acne vulgaris. clinicaltrials.gov/ct2/show/NCT00664248 (first received 22 April 2008).
NCT01106807 {published data only}
-
- NCT01106807. Exploratory study to evaluate the efficacy and safety of CD07223 gel in subjects with acne. clinicaltrials.gov/ct2/show/NCT01106807 (first received 20 April 2010).
NCT01237821 {published data only}
-
- NCT01237821. Comparing OTC acne treatment to prescription regimen. clinicaltrials.gov/ct2/show/NCT01237821 (first received 10 November 2010).
NCT01445301 {published data only}
-
- NCT01445301. Study STF115287, a clinical confirmation study of GSK2585823 in the treatment of acne vulgaris in Japanese subjects. clinicaltrials.gov/ct2/show/NCT01445301 (first received 3 October 2011).
NCT01501799 {published data only}
-
- CTRI/2011/05/001762. A clinical trial to compare adapalene and benzoyl peroxide combination local application gel to Epiduo of Galderma Laboratories, L.P., and both these to an inactive local application gel in patients with mild to severe pimple. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2011/05/001762 (first received 26 May 2011).
-
- NCT01501799. A bioequivalence study with clinical endpoints comparing adapalene and benzoyl peroxide topical gel 0.1%/2.5% (Actavis Mid‐Atlantic LLC) to Epiduo™ (adapalene and benzoyl peroxide) Gel 0.1%/2.5% (Galderma Laboratories, L.P.) in the treatment of mild to severe acne vulgaris. clinicaltrials.gov/ct2/show/NCT01501799 (first received 29 December 2011).
NCT01742637 {published data only}
-
- NCT01742637. Study comparing adapalene and benzoyl peroxide gel 0.1%/2.5% to Epiduo® and both to a placebo control in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT01742637 (first received 5 December 2012).
NCT01769235 {published data only}
-
- NCT01769235. Study comparing clindamycin phosphate and benzoyl peroxide gel to Acanya® Gel and both to a vehicle control in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT01769235 (first received 16 January 2013).
NCT01769664 {published data only}
-
- NCT01769664. A study comparing clindamycin 1%/benzoyl peroxide 5% topical gel to Duac® topical gel in the treatment of acne vulgaris. https://clinicaltrials.gov/ct2/show/NCT01769664 (first received 17 January 2013).
NCT01788384 {published data only}
-
- NCT01788384. Evaluate therapeutic equivalence and safety of two clindamycin phosphate and benzoyl peroxide gels in acne vulgaris. clinicaltrials.gov/ct2/show/NCT01788384 (first received 11 February 2013).
NCT01796665 {published data only}
-
- NCT01796665. A study to compare clindamycin phosphate and benzoyl peroxide topical gel 1.2%/2.5% to Acanya® topical gel in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT01796665 (first received 22 February 2013).
NCT02515305 {published data only}
-
- NCT02515305. Comparative safety and efficacy of two treatments in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT02515305 (first received 4 August 2015).
NCT02525549 {published data only}
-
- NCT02525549. Comparative safety and bioequivalence of two treatments in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT02525549 (first received 17 August 2015).
NCT02578043 {published data only}
-
- NCT02578043. A study comparing clindamycin and benzoyl peroxide gel 1.2%/3.75% to Onexton™ Gel in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT02578043 (first received 14 October 2015).
NCT02595034 {published data only}
-
- NCT02595034. A study CLBG and benzoyl peroxide gel 1%/5% to BenzaClin® Gel in the treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT02595034 (first received 1 November 2015).
NCT02616614 {published data only}
-
- NCT02616614. Double‐blind placebo‐controlled trial of generic clindamycin/benzoyl peroxide gel versus Onexton Gel in acne vulgaris. clinicaltrials.gov/ct2/show/NCT02616614 (first received 25 November 2015).
NCT02651220 {published data only}
-
- NCT02651220. Clinical end point study of generic adapalene and benzoyl peroxide gel versus Epiduo® Forte Gel in treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT02651220 (first received 5 January 2016).
NCT02709902 {published data only}
-
- NCT02709902. Study comparing adapalene/BP gel to EPIDUO® FORTE and both to a placebo control in treatment of acne vulgaris. clinicaltrials.gov/ct2/show/NCT02709902 (first received 8 March 2016).
NCT03393494 {published data only}
-
- NCT03393494. Bioequivalence study of two treatments in the treatment of acne vulgaris on the face. clinicaltrials.gov/ct2/show/NCT03393494 (first received 8 January 2018).
Peereboom‐Wynia 1984 {published data only}
-
- Peereboom‐Wynia JDR, Cornelissen PJG, Bernsen R. A new alcohol free preparation of benzoyl peroxide gel (Basiron) for acne vulgaris. A double blind trial. Tijdschrift voor Therapie, Geneesmiddel en Onderzoek 1984;9(10):519‐22. [CENTRAL: CN‐00334342]
Perez 2017 {published data only}
-
- Perez E, Fernandez JR, Rouzard K, Webb C, Voronkov M, Healy J, et al. SIG‐1459 and SIG‐1460: novel anti‐acne isoprenyl cysteine compounds. Journal of Investigative Dermatology 2017;137(10 Suppl 2):S267.
Priano 1993 {published data only}
-
- Priano L, Borghi S, Isola V, Grazioli I, Melzi G, Massone L. Topical spironolactone 5% versus benzoyl peroxide 5% + miconazole 2% in the therapy of acne: double‐blind, controlled study to evaluate the efficacy and the eventual systemic absorption [Spironolattone 5% verso benzoilperossido 5% + miconazolo 2%, per via topica nella terapia aell'acne: Studio controllato in doppio cieco per la valutazione di efficacia d non assorbimento transcutaneo]. Giornale Italiano di Dermatologia e Venereologia 1993;128(4):XXVII‐XXX. [CENTRAL: CN‐00351889]
Stinco 2016 {published data only}
-
- Stinco G, Piccirillo F, Valent F, Errichetti E, Meo N, Trevisan G, et al. Efficacy, tolerability, impact on quality of life and sebostatic activity of three topical preparations for the treatment of mild to moderate facial acne vulgaris. Giornale Italiano di Dermatologia e Venereologia 2016;151(3):230‐8. [CENTRAL: CN‐01158537; MEDLINE: ] - PubMed
Wokalek 1989 {published data only}
-
- Wokalek H. Open, controlled multicenter trial of 2 benzoyl peroxide preparations in the treatment of acne vulgaris. Zeitschrift fur Hautkrankheiten 1989;64(2):140‐4. [MEDLINE: ] - PubMed
References to ongoing studies
2005‐004708‐35 {published data only}
-
- 2005‐004708‐35. Multi‐centre, comparative, randomized, single‐blind, parallel group, clinical trial in phase IV for the evaluation of the subjects quality of life, the efficacy and the tolerance of Duac® gel (a gel containing clindamycin phosphate [equivalent to 1% clindamycin] and 5% benzoyl peroxide) and Differin® gel (a gel containing 0.1% adapalene) in the topical treatment of mild to moderate acne vulgaris. www.clinicaltrialsregister.eu/ctr‐search/search?query=2005‐004708‐35 (first received 22 June 2007).
2015‐002699‐26 {published data only}
-
- 2015‐002699‐26/DE. Pilot study of tolerability and effectivity following application of two combination topical acne products clindamycin 1% and 0.025% tretinoin gel (Acnatac® Gel), adapalen 0,1% and benzoyl peroxide 2,5% gel (Epiduo® Gel). www.clinicaltrialsregister.eu/ctr‐search/trial/2015‐002699‐26/DE (first received 13 August 2015).
ACTRN12609000443291 {published data only}
-
- ACTRN12609000443291. Efficacy and safety comparison of adapalene 0.1%/benzoyl peroxide 2.5% gel associated with lymecycline 300mg capsules versus adapalene 0.1%/benzoyl peroxide 2.5% vehicle gel associated with lymecycline 300mg capsules in the treatment of moderate to severe acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12609000443291 (first received 12 June 2009).
CTRI/2012/11/003127 {published data only}
-
- CTRI/2012/11/003127. A randomized, open label, active controlled, parallel group trial to compare the safety and efficacy of adapalene, benzoyl peroxide, and benzoyl peroxide‐clindamycin combination in patients with acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2012/11/003127 (first received 21 November 2012).
CTRI/2014/07/004734 {published data only}
-
- CTRI/2014/07/004734. A multiple center, parallel group study to evaluate the bioequivalence of test drug clindamycin 1% and benzoyl peroxide 5% gel of Watson and reference drug Benzaclin® (clindamycin 1% and benzoyl peroxide 5%) Gel of Dermik Labs, in treatment of subjects with acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2014/07/004734 (first received 14 July 2014).
CTRI/2015/11/006379 {published data only}
-
- CTRI/2015/11/006379. A randomized, double‐blind, multicentric, parallel‐group, active and placebo controlled, three arm clinical study to compare the efficacy and safety of clindamycin phosphate 1.2%/benzoyl peroxide 5% gel (of Cadila Healthcare Limited, India) versus DUAC® Gel (of Stiefel Laboratories, USA) versus placebo (vehicle gel) in the ratio of 2:2:1 respectively, in patients with acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2015/11/006379 (first received 26 November 2015).
CTRI/2016/04/006875 {published data only}
-
- CTRI/2016/04/006875. A comparative study of benzoyl peroxide 2.5% gel, adapalene 0.1% gel and their combination in treatment of acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2016/04/006875 (first received 27 April 2016).
CTRI/2017/09/009884 {published data only}
-
- CTRI/2017/09/009884. A multicenter, randomized, double blind, parallel, placebo controlled clinical endpoint study to determine the therapeutic equivalence of test product benzoyl peroxide 5% and clindamycin phosphate 1% gel and reference product BenzaClin Topical Gel in patients with acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/09/009884 (first received 22 September 2017).
CTRI/2017/12/010974 {published data only}
-
- CTRI/2017/12/010974. A clinical study to evaluate the efficacy and safety of Tila‐i Muhasa in the management of Busur Labaniyya (acne vulgaris). apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2017/12/010974 (first received 26 December 2017).
CTRI/2018/05/013744 {published data only}
-
- CTRI/2018/05/013744. Evaluation of safety and efficacy of hydrogen peroxide stabilized cream for treatment of mild to moderate acne vulgaris in comparison with benzoyl peroxide (BP) gel, double blinded randomized control study. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/05/013744 (first received 7 May 2018).
CTRI/2018/06/014684 {published data only}
-
- CTRI/2018/05/013744. A randomized, double blind, multicenter, three‐arm, parallel, placebo‐controlled clinical study to evaluate the bioequivalence using clinical endpoint of clindamycin phosphate 1.2% and benzoyl peroxide 5% gel (Encube Ethicals Private Limited, India) to DUAC® Gel (clindamycin phosphate 1.2% and benzoyl peroxide 5% gel) (Stiefel Laboratories, Inc., Research Triangle Park, NC 27709) in subjects with acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2018/06/014684 (first received 29 June 2018).
IRCT2017072035195N1 {published data only}
-
- IRCT2017072035195N1. Comparison of the efficacy of dapsone 5% gel plus oral doxycycline versus benzoyl peroxide 5% gel plus oral doxycycline in patients with moderate acne vulgaris referred to Rasht Razi hospital during 2017‐2018. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2017072035195N1 (first received 23 October 2017).
IRCT20170806035524N5 {published data only}
-
- IRCT20170806035524N5. Comparing efficacy of combination therapy with niosomal benzoyl peroxide 1% ‐ clindamycin 1% versus niosomal clindamycin 1% in acne vulgaris: a randomized clinical trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20170806035524N5 (first received 15 August 2018).
JPRN‐UMIN000019639 {published data only}
-
- JPRN‐UMIN000019639. Study of the utility of acute‐phase and remission maintenance therapy with 2.5% benzoyl peroxide gel for moderate or severe acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000019639 (first received 1 December 2015).
JPRN‐UMIN000024874 {published data only}
-
- JPRN‐UMIN000024874. Study of the utility of combination therapy 2.5% benzoyl peroxide gel and 2% ozenoxacin lotion for moderate or severe acne vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000024874 (first received 15 February 2017).
NCT00869492 {published data only}
-
- NCT00869492. Comparison of nadifloxacin cream alone and with benzoyl peroxide solution in the treatment of acne. clinicaltrials.gov/ct2/show/NCT00869492 (first received 26 March 2009).
NCT00877409 {published data only}
-
- NCT00877409. A monocentric, single‐blind, randomized, placebo‐controlled study of the safety and efficacy of the topical drug Acnase creme in the treatment of acne vulgaris I and II. clinicaltrials.gov/ct2/show/NCT00877409 (first received 7 April 2009).
NCT01422785 {published data only}
-
- NCT01422785. A study comparing combination clindamycin phosphate/tretinoin gel alone versus with benzoyl peroxide foaming cloths for facial acne. clinicaltrials.gov/ct2/show/NCT01422785 (first received 24 August 2011).
NCT02005666 {published data only}
-
- NCT02005666. To compare the efficacy and safety of clindamycin phosphate 1.2%/benzoyl peroxide 5% gel of CHL versus DUAC® gel. clinicaltrials.gov/ct2/show/NCT02005666 (first received 9 December 2013).
NCT02731105 {published data only}
-
- NCT02731105. Pilot study of tolerability and effectivity of two combination topical acne products (PREFECT). clinicaltrials.gov/ct2/show/NCT02731105 (first received 29 March 2016).
NCT03076320 {published data only}
-
- NCT03076320. Pirfenidone plus M‐DDO gel in moderate and severe acne. clinicaltrials.gov/ct2/show/NCT03076320 (first received 6 March 2017).
NCT03563365 {published data only}
-
- NCT03563365. The functional and emotional benefits of Replenix Power of Three with Resveratrol. clinicaltrials.gov/ct2/show/NCT03563365 (first received 20 June 2018).
Additional references
ACORN
-
- Acne Core Outcomes Research Network. sites.psu.edu/acnecoreoutcomes/ (accessed before 14 May 2018).
Archer 2012
-
- Archer CB, Cohen SN, Baron SE. Guidance on the diagnosis and clinical management of acne. Clinical & Experimental Dermatology 2012;37(Suppl 1):1‐6. [MEDLINE: ] - PubMed
Asai 2016
Bach 1993
-
- Bach M, Bach D. Psychiatric and psychometric issues in acne excoriée. Psychotherapy & Psychosomatics 1993;60(3‐4):207‐10. [MEDLINE: ] - PubMed
Barbaric 2016
Barratt 2009
-
- Barratt H, Hamilton F, Car J, Lyons C, Layton A, Majeed A. Outcome measures in acne vulgaris: systematic review. British Journal of Dermatology 2009;160(1):132‐6. [MEDLINE: ] - PubMed
Bhate 2013
-
- Bhate K, Williams HC. Epidemiology of acne vulgaris. British Journal of Dermatology 2013;168(3):474‐85. [MEDLINE: ] - PubMed
Bickers 2006
-
- Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E, et al. The burden of skin diseases: 2004 ‐ a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. Journal of the American Academy of Dermatology 2006;55(3):490‐500. [MEDLINE: ] - PubMed
Bojar 1994
-
- Bojar RA, Eady EA, Jones CE, Cunliffe WJ, Holland KT. Inhibition of erythromycin‐resistant propionibacteria on the skin of acne patients by topical erythromycin with and without zinc. British Journal of Dermatology 1994;130(3):329‐36. [MEDLINE: ] - PubMed
Bojar 1995
-
- Bojar RA, Cunliffe WJ, Holland KT. The short‐term treatment of acne vulgaris with benzoyl peroxide: effects on the surface and follicular cutaneous microflora. British Journal of Dermatology 1995;132(2):204‐8. [MEDLINE: ] - PubMed
Brown 1998
-
- Brown SK, Shalita AR. Acne vulgaris. Lancet 1998;351(9119):1871‐6. [MEDLINE: ] - PubMed
Burkhart 1999
Chivot 2005
-
- Chivot M. Retinoid therapy for acne. A comparative review. American Journal of Clinical Dermatology 2005;6(1):13‐9. [MEDLINE: ] - PubMed
COMET
-
- Core Outcome Measures in Effectiveness Trials Initiative. www.cometinitiative.org (accessed before 14 May 2018).
Cunliffe 1986
-
- Cunliffe WJ. Acne and unemployment. British Journal of Dermatology 1986;115(3):386. [MEDLINE: ] - PubMed
Cunliffe 2000
-
- Cunliffe WJ, Holland DB, Clark SM, Stables GI. Comedogenesis: some new aetiological, clinical and therapeutic strategies. British Journal of Dermatology 2000;142(6):1084‐91. [MEDLINE: ] - PubMed
Dressler 2016
-
- Dressler C, Rosumeck S, Nast A. How much do we know about maintaining treatment response after successful acne therapy? Systematic review on the efficacy and safety of acne maintenance therapy. Dermatology 2016;232:371‐80. [MEDLINE: ] - PubMed
Dréno 2010
-
- Dréno B. Recent data on epidemiology of acne. Annales de Dermatologie et de Venereologie 2010;137(Suppl 2):S49‐51. [PUBMED: 21095494] - PubMed
Dutil 2010
-
- Dutil M. Benzoyl peroxide: enhancing antibiotic efficacy in acne management. Skin Therapy Letter 2010;15(10):5‐7. [MEDLINE: ] - PubMed
Egger 1997
Fakhouri 2009
-
- Fakhouri T, Yentzer BA, Feldman SR. Advancement in benzoyl peroxide‐based acne treatment: methods to increase both efficacy and tolerability. Journal of Drugs in Dermatology 2009;8(7):657‐61. [MEDLINE: ] - PubMed
FDA 2005
-
- U.S. Department of Health and Human Services, Food, Drug Administration. Center for Drug Evaluation and Research. Guidance for Industry. Acne vulgaris: developing drugs for treatment Draft Guidance. www.fda.gov/downloads/Drugs/.../Guidances/UCM071292.pdf (accessed before 1 December 2017).
Gamble 2012
-
- Gamble R, Dunn J, Dawson A, Petersen B, McLaughlin L, Small A, et al. Topical antimicrobial treatment of acne vulgaris: an evidence‐based review. American Journal of Clinical Dermatology 2012;13(3):141‐52. [MEDLINE: ] - PubMed
Goh 2015
-
- Goh CL, Abad‐Casintahan F, Aw DC, Baba R, Chan LC, Hung NT, et al. South‐East Asia study alliance guidelines on the management of acne vulgaris in South‐East Asian patients. Journal of Dermatology 2015;42(10):945‐53. [MEDLINE: ] - PubMed
Gold 2016a
Gollnick 2003
-
- Gollnick H, Cunliffe W, Berson D, Dreno B, Finlay A, Leyden JJ, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. Journal of the American Academy of Dermatology 2003;49(Suppl 1):S1‐37. [MEDLINE: ] - PubMed
GRADEpro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Version 2015. McMaster University (developed by Evidence Prime, Inc.), 2015.
Halvorsen 2011
-
- Halvorsen JA, Stern RS, Dalgard F, Thoresen M, Bjertness E, Lien L. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: a population‐based study. Journal of Investigative Dermatology 2011;131(2):363‐70. [MEDLINE: ] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Ingram 2010
James 2005
-
- James WD. Acne. New England Journal of Medicine 2005;352(14):1463‐72. [MEDLINE: ] - PubMed
Jeremy 2003
-
- Jeremy AH, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. Journal of Investigative Dermatology 2003;121(1):20‐7. [MEDLINE: ] - PubMed
Jowett 1985
-
- Jowett S, Ryan T. Skin disease and handicap: an analysis of the impact of skin conditions. Social Science & Medicine 1985;20(4):425‐9. [MEDLINE: ] - PubMed
Katsambas 2004
-
- Katsambas AD, Stefanaki C, Cunliffe WJ. Guidelines for treating acne. Clinics in Dermatology 2004;22(5):439‐44. [MEDLINE: ] - PubMed
Kempiak 2008
-
- Kempiak SJ, Uebelhoer N. Superficial chemical peels and microdermabrasion for acne vulgaris. Seminars in Cutaneous Medicine and Surgery 2008;27(3):212‐20. [PUBMED: 18786500] - PubMed
Kolli 2019
-
- Kolli SS, Pecone D, Pona A, Cline A, Feldman SR. Topical retinoids in acne vulgaris: a systematic review. American Journal of Clinical Dermatology 2019;20(3):345‐65. [PUBMED: 30674002] - PubMed
Kraft 2011
Krakowski 2008
-
- Krakowski AC, Stendardo S, Eichenfield LF. Practical considerations in acne treatment and the clinical impact of topical combination therapy. Pediatric Dermatology 2008;25(Suppl 1):1‐14. [MEDLINE: ] - PubMed
Lamel 2015
-
- Lamel SA, Sivamani RK, Rahvar M, Maibach HI. Evaluating clinical trial design: systematic review of randomized vehicle‐controlled trials for determining efficacy of benzoyl peroxide topical therapy for acne. Archives of Dermatological Research 2015;307:757‐66. - PubMed
Law 2010
-
- Law MP, Chuh AA, Molinari N, Lee A. Acne prevalence and beyond: acne disability and its predictive factors among Chinese late adolescents in Hong Kong. Clinical & Experimental Dermatology 2010;35:16‐21. [MEDLINE: ] - PubMed
Layton 2009
-
- Layton AM, Eady EA. Benzoyl peroxide and adapalene fixed combination: a novel agent for acne. British Journal of Dermatology 2009;161(5):971‐6. [MEDLINE: ] - PubMed
Layton 2010
-
- Layton AM. Disorders of sebaceous glands. In: Burns T, Breathnach S, Cox N, Griffiths C editor(s). Rook's Textbook of Dermatology. Oxford: Wiley‐Blackwell, 2010:42.1‐42.89.
Le Cleach 2017
-
- Cleach L, Lebrun‐Vignes B, Bachelot A, Beer F, Berger P, Brugère S, et al. Guidelines for the management of acne: recommendations from a French multidisciplinary group. British Journal of Dermatology 2017;177(4):908‐13. [MEDLINE: ] - PubMed
Lehmann 2002
-
- Lehmann HP, Robinson KA, Andrews JS, Holloway V, Goodman SN. Acne therapy: a methodologic review. Journal of the American Academy of Dermatology 2002;47(2):231‐40. [MEDLINE: ] - PubMed
Levine 1983
-
- Levine RM, Rasmussen JE. Intralesional corticosteroids in the treatment of nodulocystic acne. Archives of Dermatology 1983;119(6):480‐1. [PUBMED: 6222700] - PubMed
Liberati 2009
Moher 2009
Murray 2012
-
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability‐adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2197‐223. [MEDLINE: ] - PubMed
Nast 2012
-
- Nast A, Dréno B, Bettoli V, Degitz K, Erdmann R, Finlay AY, et al. European evidence‐based (S3) guidelines for the treatment of acne. Journal of the European Academy of Dermatology and Venereology 2012;26(Suppl 1):1‐29. [MEDLINE: ] - PubMed
Nast 2016
-
- Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin Nast A, Dréno B, et al. European evidence‐based (S3) guideline for the treatment of acne ‐ update 2016 ‐ short version. Journal of the European Academy of Dermatology and Venereology 2016;30(8):1261‐8. - PubMed
Oon 2019
Patel 2010
-
- Patel M, Bowe WP, Heughebaert C, Shalita AR. The development of antimicrobial resistance due to the antibiotic treatment of acne vulgaris: a review. Journal of Drugs in Dermatology 2010;9(6):655‐64. [MEDLINE: ] - PubMed
Plot Digitizer [Computer program]
-
- http://plotdigitizer.sourceforge.net/. Accessed 30 June 2018. Plot Digitizer 2015. http://plotdigitizer.sourceforge.net/. Accessed 30 June 2018.
Rai 2013
-
- Rai R, Natarajan K. Laser and light based treatments of acne. Indian Journal of Dermatology, Venereology, & Leprology 2013;79(3):300‐9. [MEDLINE: ] - PubMed
Ramirez 2006
-
- Ramirez J, Batra RS, Miller T, Mastej J. Evaluation of in‐vivo antimicrobial efficacy of three formulations containing benzoyl peroxide on P. acnes. 64th Annual Meeting of the American Academy of Dermatology 2006. sadpas.co.za/wp‐content/uploads/2016/06/Ramirez‐Batra‐et‐al‐270.pdf (accessed before 22 May 2018).
Ramli 2012
-
- Ramli R, Malik AS, Hani AF, Jamil A. Acne analysis, grading and computational assessment methods: an overview. Skin Research & Technology 2012;18(1):1‐14. [MEDLINE: ] - PubMed
Rendon 2010
Sagransky 2009
-
- Sagransky M, Yentzer BA, Feldman SR. Benzoyl peroxide: a review of its current use in the treatment of acne vulgaris. Expert Opinion on Pharmacotherapy 2009;10(15):2555‐62. [MEDLINE: ] - PubMed
Schmitt 2016
-
- Schmitt J, Deckert S, Alam M, Apfelbacher C, Barbaric J, Bauer A, et al. Report from the kick‐off meeting of the Cochrane Skin Group Core Outcome Set Initiative (CSG‐COUSIN). British Journal of Dermatology 2016;174(2):287‐95. [MEDLINE: ] - PubMed
Schulz 2010
Schäfer 2001
-
- Schäfer T, Nienhaus A, Vieluf D, Berger J, Ring J. Epidemiology of acne in the general population: the risk of smoking. British Journal of Dermatology 2001;145(1):100‐4. [PUBMED: 11453915] - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group. Available from guidelinedevelopment.org/handbook (accessed 1 May 2018).
Seidler 2010
-
- Seidler EM, Kimball AB. Meta‐analysis comparing efficacy of benzoyl peroxide, clindamycin, benzoyl peroxide with salicylic acid, and combination benzoyl peroxide/clindamycin in acne. Journal of the American Academy of Dermatology 2010;63(1):52‐62. [MEDLINE: ] - PubMed
Simpson 2008
-
- Simpson N, Cunliffe W. Disorders of sebaceous glands. In: Burns T, Breathnach S, Cox N, Griffiths C editor(s). Rook's Textbook of Dermatology. 7th Edition. Malden, MA, USA: Wiley, Blackwell Science Ltd, 2008:43.1.
Smith 2010
-
- Smith E V, Grindlay DJ, Williams HC. What's new in acne? An analysis of systematic reviews published in 2009‐2010. Clinical & Experimental Dermatology 2010;36(2):119‐22. [MEDLINE: ] - PubMed
Strauss 2007
-
- Strauss JS, Krowchuk DP, Leyden JJ, Lucky AW, Shalita AR, Siegfried EC, et al. Guidelines of care for acne vulgaris management. Journal of the American Academy of Dermatology 2007;56(4):651‐63. [MEDLINE: ] - PubMed
Tan 2009
-
- Tan JK. Adapalene 0.1% and benzoyl peroxide 2.5%: a novel combination for treatment of acne vulgaris. Skin Therapy Letter 2009;14(6):4‐5. [MEDLINE: ] - PubMed
Tanghetti 2013
Taylor 2004
-
- Taylor GA, Shalita AR. Benzoyl peroxide‐based combination therapies for acne vulgaris: a comparative review. American Journal of Clinical Dermatology 2004;5(4):261‐5. [MEDLINE: ] - PubMed
Thiboutot 2009
-
- Thiboutot D, Gollnick H, Bettoli V, Dréno B, Kang S, Leyden JJ, et al. New insights into the management of acne: an update from the Global Alliance to Improve Outcomes in Acne Group. Journal of the American Academy of Dermatology 2009;60(5 Suppl):S1‐50. [MEDLINE: ] - PubMed
Wei 2010
-
- Wei B, Pang Y, Zhu H, Qu L, Xiao T, Wei HC, et al. The epidemiology of adolescent acne in North East China. Journal of the European Academy of Dermatology & Venereology 2010;24:953‐7. [MEDLINE: ] - PubMed
White 1998
-
- White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. Journal of the American Academy of Dermatology 1998;39:S34‐7. [MEDLINE: ] - PubMed
Williams 2012
-
- Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet 2012;379(9813):361‐72. [MEDLINE: ] - PubMed
Yentzer 2010
-
- Yentzer BA, Hick J, Reese EL, Uhas A, Feldman SR, Balkrishnan R. Acne vulgaris in the United States: a descriptive epidemiology. Cutis; Cutaneous Medicine for the Practitioner 2010;86(2):94‐9. [MEDLINE: ] - PubMed
Zaenglein 2006
-
- Zaenglein AL, Thiboutot DM. Expert committee recommendations for acne management. Pediatrics 2006;118(3):1188‐99. [MEDLINE: ] - PubMed
Zaenglein 2016
-
- Zaenglein AL, Pathy AL, Schlosser BJ, Alikhan A, Baldwin HE, Berson DS, et al. Guidelines of care for the management of acne vulgaris. Journal of the American Academy of Dermatology 2016;74(5):945‐73. [MEDLINE: ] - PubMed
Zarchi 2012
-
- Zarchi K, Jemec GBE. Severity assessment and outcome measures in acne vulgaris. Current Dermatology Reports 2012;1(3):131‐6. [EMBASE: 2012710458]
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

