Adherence to Prescribed E-Diary Recording by Patients With Seasonal Allergic Rhinitis: Observational Study
- PMID: 32175909
- PMCID: PMC7105930
- DOI: 10.2196/16642
Adherence to Prescribed E-Diary Recording by Patients With Seasonal Allergic Rhinitis: Observational Study
Abstract
Background: Complete diagnosis and therapy of seasonal allergic rhinoconjunctivitis require evidence that exposure to the sensitizing pollen triggers allergic symptoms. Electronic clinical diaries, by recording disease severity scores and pollen exposure, can demonstrate this association. However, patients who spontaneously download an e-diary app show very low adherence to their recording.
Objective: The objective of our study was to assess adherence of patients with seasonal allergic rhinitis to symptom recording via e-diary explicitly prescribed by an allergist within a blended care approach.
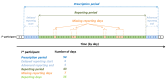
Methods: The @IT-2020 project is investigating the diagnostic synergy of mobile health and molecular allergology in patients with seasonal allergic rhinitis. In the pilot phase of the study, we recruited Italian children (Rome, Italy) and adults (Pordenone, Italy) with seasonal allergic rhinitis and instructed them to record their symptoms, medication intake, and general conditions daily through a mobile app (Allergy.Monitor) during the relevant pollen season.
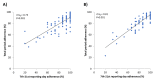
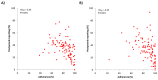
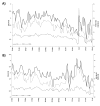
Results: Overall, we recruited 101 Italian children (Rome) and 93 adults (Pordenone) with seasonal allergic rhinitis. Adherence to device use slowly declined during monitoring in 3 phases: phase A: first week, ≥1267/1358, 90%; phase B: second to sixth week, 4992/5884, 80% to 90%; and phase C: seventh week onward, 2063/2606, 70% to 80%. At the individual level, the adherence assessed in the second and third weeks of recording predicted with enough confidence (Rome: Spearman ρ=0.75; P<.001; Pordenone: ρ=0.81; P<.001) the overall patient adherence to recording and was inversely related to postponed reporting (ρ=-0.55; P<.001; in both centers). Recording adherence was significantly higher during the peak grass pollen season in Rome, but not in Pordenone.
Conclusions: Adherence to daily recording in an e-diary, prescribed and motivated by an allergist in a blended care setting, was very high. This observation supports the use of e-diaries in addition to face-to-face visits for diagnosis and treatment of seasonal allergic rhinitis and deserves further investigation in real-life contexts.
Keywords: blended care; e-Diary; mobile health; pollen; precision medicine; seasonal allergic rhinitis.
©Marco Di Fraia, Salvatore Tripodi, Stefania Arasi, Stephanie Dramburg, Sveva Castelli, Danilo Villalta, Francesca Buzzulini, Ifigenia Sfika, Valeria Villella, Ekaterina Potapova, Serena Perna, Maria Antonia Brighetti, Alessandro Travaglini, Pierluigi Verardo, Simone Pelosi, Anna Maria Zicari, Paolo Maria Matricardi. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 16.03.2020.
Conflict of interest statement
Conflicts of Interest: PMM reports grants and personal fees from EUROIMMUN AG, during the conduct of the study; and grants and personal fees from Thermo Fisher Scientific, and personal fees from Hycor Biomedical Inc, outside the submitted work. ST is cofounder of TPS Production. S Pelosi reports personal fees from TPS Production. FB reports personal fees from Abbvie. The remaining authors declare no conflict of interest.
Figures


Similar articles
-
Validation Parameters of Patient-Generated Data for Digitally Recorded Allergic Rhinitis Symptom and Medication Scores in the @IT.2020 Project: Exploratory Study.JMIR Mhealth Uhealth. 2022 Jun 3;10(6):e31491. doi: 10.2196/31491. JMIR Mhealth Uhealth. 2022. PMID: 35657659 Free PMC article.
-
The impact of telemonitoring on adherence to nasal corticosteroid treatment in children with seasonal allergic rhinoconjunctivitis.Clin Exp Allergy. 2014 Oct;44(10):1246-54. doi: 10.1111/cea.12386. Clin Exp Allergy. 2014. PMID: 25109375 Clinical Trial.
-
Factors predicting the outcome of allergen-specific nasal provocation test in children with grass pollen allergic rhinitis.Front Allergy. 2023 May 26;4:1186353. doi: 10.3389/falgy.2023.1186353. eCollection 2023. Front Allergy. 2023. PMID: 37304166 Free PMC article.
-
Digital technologies for an improved management of respiratory allergic diseases: 10 years of clinical studies using an online platform for patients and physicians.Ital J Pediatr. 2020 Jul 25;46(1):105. doi: 10.1186/s13052-020-00870-z. Ital J Pediatr. 2020. PMID: 32711557 Free PMC article. Review.
-
The "allergic nose as a pollen detector" concept: e-Diaries to predict pollen trends.Pediatr Allergy Immunol. 2023 Jun;34(6):e13966. doi: 10.1111/pai.13966. Pediatr Allergy Immunol. 2023. PMID: 37366207 Review.
Cited by
-
The Potential of Clinical Decision Support Systems for Prevention, Diagnosis, and Monitoring of Allergic Diseases.Front Immunol. 2020 Sep 10;11:2116. doi: 10.3389/fimmu.2020.02116. eCollection 2020. Front Immunol. 2020. PMID: 33013892 Free PMC article. Review.
-
The impact of a digital wheeze detector on parental disease management of pre-school children suffering from wheezing-a pilot study.Pilot Feasibility Stud. 2021 Oct 9;7(1):185. doi: 10.1186/s40814-021-00917-w. Pilot Feasibility Stud. 2021. PMID: 34627391 Free PMC article.
-
Medication adherence in allergic diseases and asthma: a literature review.Front Pharmacol. 2024 Dec 2;15:1488665. doi: 10.3389/fphar.2024.1488665. eCollection 2024. Front Pharmacol. 2024. PMID: 39687293 Free PMC article. Review.
-
Digital Coaching Using Smart Inhaler Technology to Improve Asthma Management in Patients With Asthma in Italy: Community-Based Study.JMIR Mhealth Uhealth. 2022 Nov 2;10(11):e25879. doi: 10.2196/25879. JMIR Mhealth Uhealth. 2022. PMID: 36322120 Free PMC article.
-
Detection of Potential Arbovirus Infections and Pregnancy Complications in Pregnant Women in Jamaica Using a Smartphone App (ZIKApp): Pilot Evaluation Study.JMIR Form Res. 2022 Jul 27;6(7):e34423. doi: 10.2196/34423. JMIR Form Res. 2022. PMID: 35896029 Free PMC article.
References
-
- van Cauwenberge P, Bachert C, Passalacqua G, Bousquet J, Canonica GW, Durham SR, Fokkens WJ, Howarth PH, Lund V, Malling HJ, Mygind N, Passali D, Scadding GK, Wang DY. Consensus statement on the treatment of allergic rhinitis. European Academy of Allergology and Clinical Immunology. Allergy. 2000 Feb;55(2):116–34. doi: 10.1034/j.1398-9995.2000.00526.x. - DOI - PubMed
-
- Krzych-Fałta E, Sowa J, Wojas O, Piekarska B, Sybilski A, Samolinski B. Allergen Challenge Chamber: an innovative solution in allergic rhinitis diagnosis. Postepy Dermatol Alergol. 2015 Dec;32(6):421–5. doi: 10.5114/pdia.2015.56096. http://europepmc.org/abstract/MED/26755904 - DOI - PMC - PubMed
-
- Boelke G, Berger U, Bergmann K, Bindslev-Jensen C, Bousquet J, Gildemeister J, Jutel M, Pfaar O, Sehlinger T, Zuberbier T. Peak nasal inspiratory flow as outcome for provocation studies in allergen exposure chambers: a GALEN study. Clin Transl Allergy. 2017;7:33. doi: 10.1186/s13601-017-0169-4. https://ctajournal.biomedcentral.com/articles/10.1186/s13601-017-0169-4 - DOI - DOI - PMC - PubMed
-
- Scadding G, Hellings P, Alobid I, Bachert C, Fokkens W, van Wijk RG, Gevaert P, Guilemany J, Kalogjera L, Lund V, Mullol J, Passalacqua G, Toskala E, van Drunen C. Diagnostic tools in Rhinology EAACI position paper. Clin Transl Allergy. 2011 Jun 10;1(1):2. doi: 10.1186/2045-7022-1-2. https://ctajournal.biomedcentral.com/articles/10.1186/2045-7022-1-2 - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

