Association Between Vaccine Exemption Policy Change in California and Adverse Event Reporting
- PMID: 32176185
- PMCID: PMC7874304
- DOI: 10.1097/INF.0000000000002585
Association Between Vaccine Exemption Policy Change in California and Adverse Event Reporting
Abstract
Background: California Senate Bill 277 (SB277) eliminated non-medical immunization exemptions. Since its introduction on February 19, 2015, the rate of medical exemptions in the state has increased. Filing a report to Vaccine Adverse Event Reporting System (VAERS) may be perceived as helpful in applying for a medical exemption. Our objective was to describe trends in reporting to VAERS from California coincident with introduction of SB277.
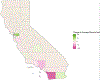
Methods: This was a retrospective study of Californian children <18 years for whom a VAERS report was submitted between June 1, 2011 and July 31, 2018. VAERS is a national, passive, vaccine safety surveillance program co-managed by Centers for Disease Control and Prevention and FDA. The main outcomes were the proportion of VAERS reports submitted by parents (vs. other reporter types), time from immunization to VAERS report (reporting time), and adverse event type. We also performed spatial analysis, mapping reports pre- and post-mandate by county.
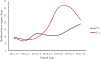
Results: We identified 6703 VAERS reports from California during the study period. The proportion of reports received from parents increased after implementation of SB277, from 14% to 23%. The median reporting time by parents increased from 9 days in 2013-2014 to 31 days in 2016-2017. After the introduction of SB277, we observed an increase in reports describing behavioral and developmental symptoms among reports submitted >6 months after immunization.
Conclusions: These recent changes in reporting patterns coincident with the introduction of SB277 may indicate that more parents are using VAERS to assist in applying for a medical exemption for their child.
Conflict of interest statement
Conflicts of interest
No potential conflicts of interest were reported by the authors
Figures
References
-
- Educational and Child Care Facility Immunization Requirements. California State Congress; 2015:120325–120380.
-
- Medical Dictionary for Regulatory Activities. https://www.meddra.org/how-to-use/support-documentation. Published 2019. Accessed May 7, 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

