Complement Activation Levels Are Related to Disease Stage in AMD
- PMID: 32176267
- PMCID: PMC7401663
- DOI: 10.1167/iovs.61.3.18
Complement Activation Levels Are Related to Disease Stage in AMD
Abstract
Purpose: To study the levels of complement activation in different disease stages of AMD and the influence of genetic polymorphisms in complement genes.
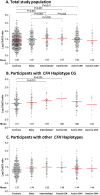
Methods: We included 797 patients with AMD and 945 controls from the European Genetic Database. Patients were grouped into five AMD stages: early AMD, intermediate AMD, central geographic atrophy, active choroidal neovascularization or inactive choroidal neovascularization. Differences in complement activation, as defined by the systemic C3d/C3 ratio, between AMD stages were evaluated using general linear modeling. In addition, we evaluated the influence of 18 genetic AMD polymorphisms in complement genes and their effect on complement activation. Differences in complement activation between stages were evaluated stratifying by complement associated haplotypes.
Results: Complement activation levels differed significantly between AMD disease stages. As compared with controls, the C3d/C3 ratio was higher in patients with intermediate AMD (P < 0.001) and central geographic atrophy (P = 0.001). Two polymorphisms in CFH (rs10922109 and rs570618) and one in CFB (rs116503776) were significantly associated with complement activation. The association between AMD disease stage and complement activation was more pronounced in patients with haplotypes associated with the highest complement activation.
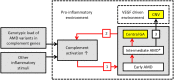
Conclusions: In general, consecutive AMD disease stages showed increasing levels of complement activation, especially in individuals with a genetic burden in complement genes. These findings contribute to the discussion on the pathogenesis of AMD in relation to complement activation and might suggest refinement in patient selection and the optimum window of treatment with complement inhibitors. Prospective studies are needed to confirm these results.
Conflict of interest statement
Disclosure:
Figures
References
-
- Joachim N, Mitchell P, Burlutsky G, Kifley A, Wang JJ. The incidence and progression of age-related macular degeneration over 15 years: the Blue Mountains Eye Study. Ophthalmology. 2015; 122: 2482–2489. - PubMed
-
- de Jong PT. Age-related macular degeneration. N Engl J Med. 2006; 355: 1474–1485. - PubMed
-
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002; 134: 411–431. - PubMed
-
- Johnson LV, Ozaki S, Staples MK, Erickson PA, Anderson DH. A potential role for immune complex pathogenesis in drusen formation. Exp Eye Res. 2000; 70: 441–449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

