Inhibition of complement C1s in patients with cold agglutinin disease: lessons learned from a named patient program
- PMID: 32176765
- PMCID: PMC7094024
- DOI: 10.1182/bloodadvances.2019001321
Inhibition of complement C1s in patients with cold agglutinin disease: lessons learned from a named patient program
Abstract
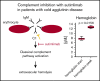
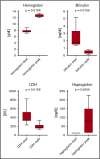
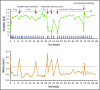
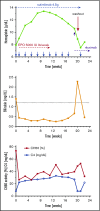
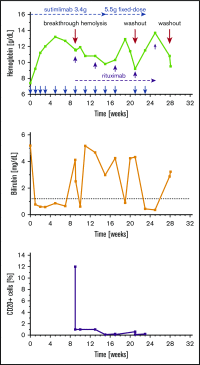
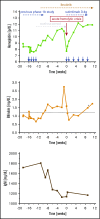
Cold agglutinin disease (CAD) causes predominantly extravascular hemolysis and anemia via complement activation. Sutimlimab is a novel humanized monoclonal antibody directed against classical pathway complement factor C1s. We aimed to evaluate the safety and efficacy of long-term maintenance treatment with sutimlimab in patients with CAD. Seven CAD patients treated with sutimlimab as part of a phase 1B study were transitioned to a named patient program. After a loading dose, patients received biweekly (once every 2 weeks) infusions of sutimlimab at various doses. When a patient's laboratory data showed signs of breakthrough hemolysis, the dose of sutimlimab was increased. Three patients started with a dose of 45 mg/kg, another 3 with 60 mg/kg, and 1 with a fixed dose of 5.5 g every other week. All CAD patients responded to re-treatment, and sutimlimab increased hemoglobin from a median initial level of 7.7 g/dL to a median peak of 12.5 g/dL (P = .016). Patients maintained near normal hemoglobin levels except for a few breakthrough events that were related to underdosing and which resolved after the appropriate dose increase. Four of the patients included were eventually treated with a biweekly 5.5 g fixed-dose regimen of sutimlimab. None of them had any breakthrough hemolysis. All patients remained transfusion free while receiving sutimlimab. There were no treatment-related serious adverse events. Overlapping treatment with erythropoietin, rituximab, or ibrutinib in individual patients was safe and did not cause untoward drug interactions. Long-term maintenance treatment with sutimlimab was safe, effectively inhibited hemolysis, and significantly increased hemoglobin levels in re-exposed, previously transfusion-dependent CAD patients.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: U.G. received personal fees from True North Therapeutics, Amgen, Takeda, and AbbVie and grants and personal fees from Roche, Celgene, Gilead, Novartis, and True North Therapeutics. J.C.G. was an employee and stockholder of True North Therapeutics. B.J. received reimbursement for travel costs and scientific advice from True North Therapeutics. The remaining authors declare no competing financial interests.
Figures
References
-
- Pruzanski W, Shumak KH. Biologic activity of cold-reacting autoantibodies (first of two parts). N Engl J Med. 1977;297(10):538-542. - PubMed
-
- Berentsen S, Ulvestad E, Langholm R, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91(4):460-466. - PubMed
-
- Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122(7):1114-1121. - PubMed
-
- Lechner K, Jäger U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116(11):1831-1838. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

