Chronic Alcohol Dysregulates Skeletal Muscle Myogenic Gene Expression after Hind Limb Immobilization in Female Rats
- PMID: 32178412
- PMCID: PMC7175129
- DOI: 10.3390/biom10030441
Chronic Alcohol Dysregulates Skeletal Muscle Myogenic Gene Expression after Hind Limb Immobilization in Female Rats
Abstract
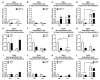
Alcohol use and aging are risk factors for falls requiring immobilization and leading to skeletal muscle atrophy. Skeletal muscle regeneration is integral to post-immobilization recovery. This study aimed to elucidate the effects of alcohol and ovarian hormone loss on the expression of genes implicated in muscle regeneration. Three-month-old female rats received an ovariectomy or a sham surgery, consumed an alcohol-containing or control diet for 10 weeks, were subjected to unilateral hind limb immobilization for seven days, and finally were allowed a three (3d)- or 14 (14d)-day recovery. Immobilization decreased the quadriceps weight at 3d and 14d, and alcohol decreased the quadriceps weight at 14d in the nonimmobilized hind limb (NI). At 3d, alcohol decreased gene expression of myoblast determination protein (MyoD) in the immobilized hind limb (IMM) and myocyte enhancer factor (Mef)2C and tumor necrosis factor (TNF)α in NI, and ovariectomy increased MyoD and decreased TNFα expression in NI. At 14d, alcohol increased the gene expression of Mef2C, MyoD, TNFα, and transforming growth factor (TFG)β in IMM and decreased monocyte chemoattractant protein (MCP)1 expression in NI; ovariectomy increased TNFα expression in NI, and alcohol and ovariectomy together increased Mef2C expression in NI. Despite increased TGFβ expression, there was no concomitant alcohol-mediated increase in collagen in IMM at 14d. Overall, these data indicate that alcohol dysregulated the post-immobilization alteration in the expression of genes implicated in regeneration. Whether alcohol-mediated molecular changes correspond with post-immobilization functional alterations remains to be determined.
Keywords: ethanol; immobilization; inflammation; ovarian hormone loss; ovariectomy; recovery; regeneration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Treatment with risedronate or alendronate prevents hind-limb immobilization-induced loss of bone density and strength in adult female rats.Bone. 2000 Nov;27(5):639-45. doi: 10.1016/s8756-3282(00)00375-6. Bone. 2000. PMID: 11062350
-
Reloading Promotes Recovery of Disuse Muscle Loss by Inhibiting TGFβ Pathway Activation in Rats After Hind Limb Suspension.Am J Phys Med Rehabil. 2017 Jun;96(6):430-437. doi: 10.1097/PHM.0000000000000617. Am J Phys Med Rehabil. 2017. PMID: 27610551
-
Muscle damaging eccentric exercise attenuates disuse-induced declines in daily myofibrillar protein synthesis and transiently prevents muscle atrophy in healthy men.Am J Physiol Endocrinol Metab. 2021 Nov 1;321(5):E674-E688. doi: 10.1152/ajpendo.00294.2021. Epub 2021 Oct 11. Am J Physiol Endocrinol Metab. 2021. PMID: 34632796 Free PMC article.
-
Ovarian hormone status and skeletal muscle inflammation during recovery from disuse in rats.Exp Physiol. 2007 Jan;92(1):219-32. doi: 10.1113/expphysiol.2006.035071. Epub 2006 Sep 21. Exp Physiol. 2007. PMID: 16990367
-
Regrowth of skeletal muscle atrophied from inactivity.Med Sci Sports Exerc. 2004 Jan;36(1):52-9. doi: 10.1249/01.MSS.0000106175.24978.84. Med Sci Sports Exerc. 2004. PMID: 14707768 Review.
Cited by
-
Episodic Binge-like Ethanol Reduces Skeletal Muscle Strength Associated with Atrophy, Fibrosis, and Inflammation in Young Rats.Int J Mol Sci. 2023 Jan 14;24(2):1655. doi: 10.3390/ijms24021655. Int J Mol Sci. 2023. PMID: 36675170 Free PMC article.
-
IL-1β and TNF-α Modulation of Proliferated and Committed Myoblasts: IL-6 and COX-2-Derived Prostaglandins as Key Actors in the Mechanisms Involved.Cells. 2020 Sep 1;9(9):2005. doi: 10.3390/cells9092005. Cells. 2020. PMID: 32882817 Free PMC article.
-
Alcohol amplifies cingulate cortex signaling and facilitates immobilization-induced hyperalgesia in female rats.Neurosci Lett. 2021 Sep 14;761:136119. doi: 10.1016/j.neulet.2021.136119. Epub 2021 Jul 17. Neurosci Lett. 2021. PMID: 34280506 Free PMC article.
-
Skeletal muscle bioenergetic health and function in people living with HIV: association with glucose tolerance and alcohol use.Am J Physiol Regul Integr Comp Physiol. 2021 Nov 1;321(5):R781-R790. doi: 10.1152/ajpregu.00197.2021. Epub 2021 Sep 29. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 34585616 Free PMC article.
-
Alcohol, Resistance Exercise, and mTOR Pathway Signaling: An Evidence-Based Narrative Review.Biomolecules. 2022 Dec 20;13(1):2. doi: 10.3390/biom13010002. Biomolecules. 2022. PMID: 36671386 Free PMC article. Review.
References
-
- Lukaszyk C., Harvey L., Sherrington C., Keay L., Tiedemann A., Coombes J., Clemson L., Ivers R. Risk factors, incidence, consequences and prevention strategies for falls and fall-injury within older indigenous populations: a systematic review. Aust. New Zealand J. Public Heal. 2016;40:564–568. doi: 10.1111/1753-6405.12585. - DOI - PubMed
-
- Honkanen R., Visuri T. Blood alcohol levels in a series of injured patients with special reference to accident and type of injury. Ann. Chir. Gynaecol. 1976;65:287–294. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

