Reversible Cross-Linked Mixed Micelles for pH Triggered Swelling and Redox Triggered Degradation for Enhanced and Controlled Drug Release
- PMID: 32178423
- PMCID: PMC7151195
- DOI: 10.3390/pharmaceutics12030258
Reversible Cross-Linked Mixed Micelles for pH Triggered Swelling and Redox Triggered Degradation for Enhanced and Controlled Drug Release
Abstract
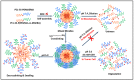
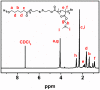
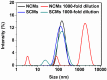
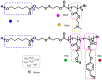
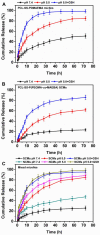
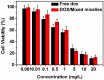
Good stability and controlled drug release are important properties of polymeric micelles for drug delivery. A good candidate for drug delivery must have outstanding stability in a normal physiological environment, followed with low drug leakage and side effects. Moreover, the chemotherapeutic drug in the micellar core should also be quickly and "on-demand" released in the intracellular microenvironment at the tumor site, which is in favor of overcoming multidrug resistance (MDR) effects of tumor cells. In this work, a mixed micelle was prepared by the simple mix of two amphiphilic copolymers, namely PCL-SS-P(PEGMA-co-MAEBA) and PCL-SS-PDMAEMA, in aqueous solution. In the mixed micelle's core-shell structure, PCL blocks were used as the hydrophobic core, while the micellar hydrophilic shell consisted of two blocks, namely P(PEGMA-co-MAEBA) and PDMAEMA. In the micellar shell, PEGMA provided hydrophilicity and stability, while MAEBA introduced the aldehyde sites for reversible crosslinking. Meanwhile, the PDMAEMA blocks were also introduced in the micellar shell for pH-responding protonation and swelling of the micelle. The disulfide bonds between the hydrophobic core and hydrophilic shell had redox sensitive properties. Reversible cross-linked micelles (RCLMs) were obtained by crosslinking the micellar shell with an imine structure. RCLMs showed good stability and excellent ability against extensive dilution by aqueous solution. In addition, the stability in different conditions with various pH values and glutathione (GSH) concentrations was studied. Then, the anticancer drug doxorubicin (DOX) was selected as the model drug to evaluate drug entrapment and release capacity of mixed micelles. The in vitro release profiles indicated that this RCLM had controlled drug release. In the simulated normal physiological environment (pH 7.4), the drug release of the RCLMs was restrained obviously, and the cumulative drug release content was only 25.7 during 72 h. When it came to acidic conditions (pH 5.0), de-crosslinking of the micelles occurred, as well as protonation of PDMAEMA blocks and micellar swelling at the same time, which enhanced the drug release to a large extent (81.4%, 72 h). Moreover, the drug release content was promoted further in the presence of the reductant GSH. In the condition of pH 5.0 with 10 mM GSH, disulfide bonds broke-up between the micelle core and shell, followed by shedding of the shell from the inner core. Then, the micellar disassembly (degradation) happened based on the de-crosslinking and swelling, and the drug release was as high as 95.3%. The MTT assay indicated that the CLSMs showed low cytotoxicity and good biocompatibility against the HepG2 cells. In contrast, the DOX-loaded CLSMs could efficiently restrain the proliferation of tumor cells, and the cell viability after 48 h incubation was just 13.2%, which was close to that of free DOX. This reversible cross-linked mixed micelle with pH/redox responsive behaviors is a potential nanocarrier for chemotherapy.
Keywords: drug delivery; mixed micelles; reversible cross-link; stimulus-responsive.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Smart pH-sensitive micelles based on redox degradable polymers as DOX/GNPs carriers for controlled drug release and CT imaging.Colloids Surf B Biointerfaces. 2018 Mar 1;163:29-40. doi: 10.1016/j.colsurfb.2017.12.008. Epub 2017 Dec 8. Colloids Surf B Biointerfaces. 2018. PMID: 29278801
-
Dual pH/Redox-Responsive Mixed Polymeric Micelles for Anticancer Drug Delivery and Controlled Release.Pharmaceutics. 2019 Apr 11;11(4):176. doi: 10.3390/pharmaceutics11040176. Pharmaceutics. 2019. PMID: 30978912 Free PMC article.
-
Poly(ethylene glycol) shell-sheddable TAT-modified core cross-linked nano-micelles: TAT-enhanced cellular uptake and lysosomal pH-triggered doxorubicin release.Colloids Surf B Biointerfaces. 2020 Apr;188:110772. doi: 10.1016/j.colsurfb.2020.110772. Epub 2020 Jan 20. Colloids Surf B Biointerfaces. 2020. PMID: 31999965
-
A Meticulous Focus on the Determination of Critical Micelle Concentration Employing Fluorescence Spectroscopy.J Fluoresc. 2025 Feb 19. doi: 10.1007/s10895-025-04209-x. Online ahead of print. J Fluoresc. 2025. PMID: 39969713 Review.
-
Micellar delivery systems of bioactive compounds for precision nutrition.Adv Food Nutr Res. 2024;112:89-145. doi: 10.1016/bs.afnr.2024.05.009. Epub 2024 Jun 11. Adv Food Nutr Res. 2024. PMID: 39218509 Review.
Cited by
-
Thermo/pH dual-responsive micelles based on the host-guest interaction between benzimidazole-terminated graft copolymer and β-cyclodextrin-functionalized star block copolymer for smart drug delivery.J Nanobiotechnology. 2022 Feb 22;20(1):91. doi: 10.1186/s12951-022-01290-3. J Nanobiotechnology. 2022. PMID: 35193612 Free PMC article.
-
Synthetic organic materials for targeting immunotherapies to lymph nodes.Chem Mater. 2024 Oct 8;36(19):9031-9045. doi: 10.1021/acs.chemmater.4c00947. Epub 2024 Aug 30. Chem Mater. 2024. PMID: 40405914
-
Advances in Organosulfur-Based Polymers for Drug Delivery Systems.Polymers (Basel). 2024 Apr 25;16(9):1207. doi: 10.3390/polym16091207. Polymers (Basel). 2024. PMID: 38732676 Free PMC article. Review.
-
Structural Determinants of Stimuli-Responsiveness in Amphiphilic Macromolecular Nano-assemblies.Prog Polym Sci. 2024 Jan;148:101765. doi: 10.1016/j.progpolymsci.2023.101765. Epub 2023 Dec 9. Prog Polym Sci. 2024. PMID: 38476148 Free PMC article.
-
Tween 80-Based Self-Assembled Mixed Micelles Boost Valsartan Transdermal Delivery.Pharmaceuticals (Basel). 2023 Dec 22;17(1):19. doi: 10.3390/ph17010019. Pharmaceuticals (Basel). 2023. PMID: 38256853 Free PMC article.
References
-
- Yu C., Tan X., Xu Z., Zhu G., Teng W., Zhao Q., Liang Z., Wu Z., Xiong D. Smart drug carrier based on polyurethane material for enhanced and controlled DOX release triggered by redox stimulus. React. Funct. Polym. 2020;148:104507. doi: 10.1016/j.reactfunctpolym.2020.104507. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

