Genome-Wide microRNA Profiling Using Oligonucleotide Microarray Reveals Regulatory Networks of microRNAs in Nicotiana benthamiana During Beet Necrotic Yellow Vein Virus Infection
- PMID: 32178444
- PMCID: PMC7150760
- DOI: 10.3390/v12030310
Genome-Wide microRNA Profiling Using Oligonucleotide Microarray Reveals Regulatory Networks of microRNAs in Nicotiana benthamiana During Beet Necrotic Yellow Vein Virus Infection
Abstract
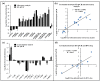
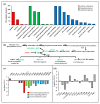
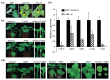
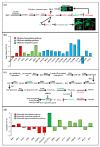
Beet necrotic yellow vein virus (BNYVV) infections induce stunting and leaf curling, as well as root and floral developmental defects and leaf senescence in Nicotiana benthamiana. A microarray analysis with probes capable of detecting 1596 candidate microRNAs (miRNAs) was conducted to investigate differentially expressed miRNAs and their targets upon BNYVV infection of N. benthamiana plants. Eight species-specific miRNAs of N. benthamiana were identified. Comprehensive characterization of the N. benthamiana microRNA profile in response to the BNYVV infection revealed that 129 miRNAs were altered, including four species-specific miRNAs. The targets of the differentially expressed miRNAs were predicted accordingly. The expressions of miR164, 160, and 393 were up-regulated by BNYVV infection, and those of their target genes, NAC21/22, ARF17/18, and TIR, were down-regulated. GRF1, which is a target of miR396, was also down-regulated. Further genetic analysis of GRF1, by Tobacco rattle virus-induced gene silencing, assay confirmed the involvement of GRF1 in the symptom development during BNYVV infection. BNYVV infection also induced the up-regulation of miR168 and miR398. The miR398 was predicted to target umecyanin, and silencing of umecyanin could enhance plant resistance against viruses, suggesting the activation of primary defense response to BNYVV infection in N. benthamiana. These results provide a global profile of miRNA changes induced by BNYVV infection and enhance our understanding of the mechanisms underlying BNYVV pathogenesis.
Keywords: Beet necrotic yellow vein virus; Nicotiana benthamiana; defense; hormone signaling; microRNAs; microarray; reactive oxygen intermediates; superoxide free radicals O2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Deep sequencing-based transcriptome profiling reveals comprehensive insights into the responses of Nicotiana benthamiana to beet necrotic yellow vein virus infections containing or lacking RNA4.PLoS One. 2014 Jan 9;9(1):e85284. doi: 10.1371/journal.pone.0085284. eCollection 2014. PLoS One. 2014. PMID: 24416380 Free PMC article.
-
The hrpZ gene of Pseudomonas syringae pv. phaseolicola enhances resistance to rhizomania disease in transgenic Nicotiana benthamiana and sugar beet.PLoS One. 2011 Mar 4;6(3):e17306. doi: 10.1371/journal.pone.0017306. PLoS One. 2011. PMID: 21394206 Free PMC article.
-
Characterization of microRNAs of Beta macrocarpa and their responses to Beet necrotic yellow vein virus infection.PLoS One. 2017 Oct 16;12(10):e0186500. doi: 10.1371/journal.pone.0186500. eCollection 2017. PLoS One. 2017. PMID: 29036205 Free PMC article.
-
Detection and characterization of spontaneous internal deletion mutants of Beet Necrotic yellow vein virus RNA3 from systemic host Nicotiana benthamiana.Virol J. 2011 Jul 1;8:335. doi: 10.1186/1743-422X-8-335. Virol J. 2011. PMID: 21718549 Free PMC article.
-
The role of reactive oxygen species in plant-virus interactions.Plant Cell Rep. 2024 Jul 16;43(8):197. doi: 10.1007/s00299-024-03280-1. Plant Cell Rep. 2024. PMID: 39014054 Review.
Cited by
-
Genetic Dissection of ToLCNDV Resistance in Resistant Sources of Cucumis melo.Int J Mol Sci. 2024 Aug 15;25(16):8880. doi: 10.3390/ijms25168880. Int J Mol Sci. 2024. PMID: 39201567 Free PMC article.
-
A Novel miRNA in Rice Associated with the Low Seed Setting Rate Symptom of Rice Stripe Virus.Int J Mol Sci. 2023 Feb 12;24(4):3675. doi: 10.3390/ijms24043675. Int J Mol Sci. 2023. PMID: 36835087 Free PMC article.
-
Overexpression of Sly-miR398b Compromises Disease Resistance against Botrytis cinerea through Regulating ROS Homeostasis and JA-Related Defense Genes in Tomato.Plants (Basel). 2023 Jul 7;12(13):2572. doi: 10.3390/plants12132572. Plants (Basel). 2023. PMID: 37447133 Free PMC article.
-
Transcriptional and post-transcriptional regulation of RNAi-related gene expression during plant-virus interactions.Stress Biol. 2022 Aug 19;2(1):33. doi: 10.1007/s44154-022-00057-y. Stress Biol. 2022. PMID: 37676459 Free PMC article. Review.
-
MicroRNA398: A Master Regulator of Plant Development and Stress Responses.Int J Mol Sci. 2022 Sep 16;23(18):10803. doi: 10.3390/ijms231810803. Int J Mol Sci. 2022. PMID: 36142715 Free PMC article. Review.
References
-
- Tamada T., Baba T. Beet necrotic yellow vein virus from rizomania-affected sugar beet in Japan. Jpn. J. Phytopathol. 1973;39:325–332. doi: 10.3186/jjphytopath.39.325. - DOI
-
- Tamada T., Shirako Y., Abe H., Saito M., Kiguchi T., Harada T. Production and pathogenicity of isolates of Beet necrotic yellow vein virus with different numbers of RNA components. J. Gen. Virol. 1989;70:3399–3409. doi: 10.1099/0022-1317-70-12-3399. - DOI
-
- Richards K.E., Tamada T. Mapping functions on the multipartite genome of Beet necrotic yellow vein virus. Annu. Rev. Phytopathol. 1992;30:291–313. doi: 10.1146/annurev.py.30.090192.001451. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

