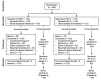
Rabies Vaccination of 6-Week-Old Puppies Born to Immunized Mothers: A Randomized Controlled Trial in a High-Mortality Population of Owned, Free-Roaming Dogs
- PMID: 32178448
- PMCID: PMC7157201
- DOI: 10.3390/tropicalmed5010045
Rabies Vaccination of 6-Week-Old Puppies Born to Immunized Mothers: A Randomized Controlled Trial in a High-Mortality Population of Owned, Free-Roaming Dogs
Abstract
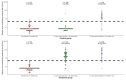
To achieve global elimination of human rabies from dogs by 2030, evidence-based strategies for effective dog vaccination are needed. Current guidelines recommend inclusion of dogs younger than 3 months in mass rabies vaccination campaigns, although available vaccines are only recommended for use by manufacturers in older dogs, ostensibly due to concerns over interference of maternally-acquired immunity with immune response to the vaccine. Adverse effects of vaccination in this age group of dogs have also not been adequately assessed under field conditions. In a single-site, owner-blinded, randomized, placebo-controlled trial in puppies born to mothers vaccinated within the previous 18 months in a high-mortality population of owned, free-roaming dogs in South Africa, we assessed immunogenicity and effect on survival to all causes of mortality of a single dose of rabies vaccine administered at 6 weeks of age. We found that puppies did not have appreciable levels of maternally-derived antibodies at 6 weeks of age (geometric mean titer 0.065 IU/mL, 95% CI 0.061-0.069; n = 346), and that 88% (95% CI 80.7-93.3) of puppies vaccinated at 6 weeks had titers ≥0.5 IU/mL 21 days later (n = 117). Although the average effect of vaccination on survival was not statistically significant (hazard ratio [HR] 1.35, 95% CI 0.83-2.18), this effect was modified by sex (p = 0.02), with the HR in females 3.09 (95% CI 1.24-7.69) and the HR in males 0.79 (95% CI 0.41-1.53). We speculate that this effect is related to the observed survival advantage that females had over males in the unvaccinated group (HR 0.27; 95% CI 0.11-0.70), with vaccination eroding this advantage through as-yet-unknown mechanisms.
Keywords: immunogenicity; maternally-acquired immunity; mortality; nonspecific effects of vaccines; sex.
Conflict of interest statement
D.L.K. received a research award at RUSVM in 2016, sponsored by Zoetis. J.E.C. received payment through Continuing Education at University of Pretoria Trust for presenting continuing professional development training to veterinarians, sponsored by Zoetis. Other authors declare no conflicts of interest.
Figures

References
-
- Food and Agriculture Organization of the United Nations. World Organisation for Animal Health. World Health Organization and Global Alliance for Rabies Control . Zero by 30: The Global Strategic Plan to End Human Deaths from Dog-Mediated Rabies by 2030. WHO/FAO/OIE; Geneva, Switzerland: 2018. [(accessed on 28 November 2019)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/272756/9789241513838-en....
-
- World Health Organization . WHO Expert Consultation on Rabies. WHO; Geneva, Switzerland: 2018. pp. 81–85. (WHO Technical Report Series No. 1012). Third Report.
-
- World Organisation for Animal Health . Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. OIE; Paris, France: 2018. Rabies (infection with rabies virus and other lyssaviruses) pp. 598–612.
LinkOut - more resources
Full Text Sources

