Antifungal Drugs
- PMID: 32178468
- PMCID: PMC7143493
- DOI: 10.3390/metabo10030106
Antifungal Drugs
Abstract
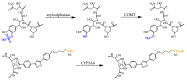
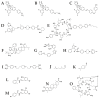
We reviewed the licensed antifungal drugs and summarized their mechanisms of action, pharmacological profiles, and susceptibility to specific fungi. Approved antimycotics inhibit 1,3-β-d-glucan synthase, lanosterol 14-α-demethylase, protein, and deoxyribonucleic acid biosynthesis, or sequestrate ergosterol. Their most severe side effects are hepatotoxicity, nephrotoxicity, and myelotoxicity. Whereas triazoles exhibit the most significant drug-drug interactions, echinocandins exhibit almost none. The antifungal resistance may be developed across most pathogens and includes drug target overexpression, efflux pump activation, and amino acid substitution. The experimental antifungal drugs in clinical trials are also reviewed. Siderophores in the Trojan horse approach or the application of siderophore biosynthesis enzyme inhibitors represent the most promising emerging antifungal therapies.
Keywords: amphotericin B; antifungal drugs; echinocandins; flucytosine; invasive fungal infections; resistance; siderophores; triazoles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hidden Crisis: How 150 People Die Every Hour from Fungal Infection While the World Turns a Blind Eye. [(accessed on 3 March 2020)]; Available online: https://www.gaffi.org/wp-content/uploads/GAFFI-Leaflet-June-2016-DWD-hid....
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

