Characterization of Human NK Cell-Derived Exosomes: Role of DNAM1 Receptor In Exosome-Mediated Cytotoxicity Against Tumor
- PMID: 32178479
- PMCID: PMC7140072
- DOI: 10.3390/cancers12030661
Characterization of Human NK Cell-Derived Exosomes: Role of DNAM1 Receptor In Exosome-Mediated Cytotoxicity Against Tumor
Abstract
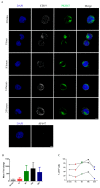
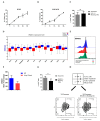
Despite the pivotal role of natural killer (NK) cells in defenses against tumors, their exploitation in cancer treatment is still limited due to their reduced ability to reaching tumor sites and the inhibitory effects of tumor microenvironment (TME) on their function. In this study, we have characterized the exosomes from IL2- or IL15-cultured human NK cells. Both cytokines induced comparable amounts of exosomes with similar cargo composition. Analysis of molecules contained within or exposed at the exosome surface, allowed the identification of molecules playing important roles in the NK cell function including IFN-γ, Lymphocyte Function-Associated Antigen (LFA-1), DNAX Accessory Molecule-1 (DNAM1) and Programmed Cell Death Protein (PD-1). Importantly, we show that DNAM1 is involved in exosome-mediated cytotoxicity as revealed by experiments using blocking antibodies to DNAM1 or DNAM1 ligands. In addition, antibody-mediated inhibition of exosome cytotoxicity results in a delay in target cell apoptosis. We also provide evidence that NK-exosomes may exert their cytolytic activity after short time interval and even at low concentrations. Regarding their possible use in immunotherapy, NK exosomes, detectable in peripheral blood, can diffuse into tissues and exert their cytolytic effect at tumor sites. This property offers a clue to integrate cancer treatments with NK exosomes.
Keywords: DNAM1; NK cells; cancer; cytotoxicity; exosomes; tumor therapy.
Conflict of interest statement
The authors declare no conflict of interest
Figures


References
-
- Moretta L., Ciccone E., Mingari M.C., Biassoni R., Moretta A. Human natural killer cells: Origin, clonality, specificity, and receptors. Adv. Immunol. 1994;55:341–380. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

