In vitro and numerical simulation of blood removal from cerebrospinal fluid: comparison of lumbar drain to Neurapheresis therapy
- PMID: 32178689
- PMCID: PMC7077023
- DOI: 10.1186/s12987-020-00185-5
In vitro and numerical simulation of blood removal from cerebrospinal fluid: comparison of lumbar drain to Neurapheresis therapy
Abstract
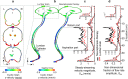
Background: Blood removal from cerebrospinal fluid (CSF) in post-subarachnoid hemorrhage patients may reduce the risk of related secondary brain injury. We formulated a computational fluid dynamics (CFD) model to investigate the impact of a dual-lumen catheter-based CSF filtration system, called Neurapheresis™ therapy, on blood removal from CSF compared to lumbar drain.
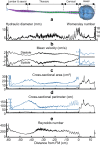
Methods: A subject-specific multiphase CFD model of CSF system-wide solute transport was constructed based on MRI measurements. The Neurapheresis catheter geometry was added to the model within the spinal subarachnoid space (SAS). Neurapheresis flow aspiration and return rate was 2.0 and 1.8 mL/min, versus 0.2 mL/min drainage for lumbar drain. Blood was modeled as a bulk fluid phase within CSF with a 10% initial tracer concentration and identical viscosity and density as CSF. Subject-specific oscillatory CSF flow was applied at the model inlet. The dura and spinal cord geometry were considered to be stationary. Spatial-temporal tracer concentration was quantified based on time-average steady-streaming velocities throughout the domain under Neurapheresis therapy and lumbar drain. To help verify CFD results, an optically clear in vitro CSF model was constructed with fluorescein used as a blood surrogate. Quantitative comparison of numerical and in vitro results was performed by linear regression of spatial-temporal tracer concentration over 24-h.
Results: After 24-h, tracer concentration was reduced to 4.9% under Neurapheresis therapy compared to 6.5% under lumbar drain. Tracer clearance was most rapid between the catheter aspiration and return ports. Neurapheresis therapy was found to have a greater impact on steady-streaming compared to lumbar drain. Steady-streaming in the cranial SAS was ~ 50× smaller than in the spinal SAS for both cases. CFD results were strongly correlated with the in vitro spatial-temporal tracer concentration under Neurapheresis therapy (R2 = 0.89 with + 2.13% and - 1.93% tracer concentration confidence interval).
Conclusion: A subject-specific CFD model of CSF system-wide solute transport was used to investigate the impact of Neurapheresis therapy on tracer removal from CSF compared to lumbar drain over a 24-h period. Neurapheresis therapy was found to substantially increase tracer clearance compared to lumbar drain. The multiphase CFD results were verified by in vitro fluorescein tracer experiments.
Keywords: Cerebrospinal fluid filtration; Computational fluid dynamics; In-vitro model; Multiphase simulation; Neurapheresis therapy; Subarachnoid hemorrhage.
Conflict of interest statement
BAM has received research funding from Biogen Inc., KBR Wyle, Alcyone Lifesciences Inc., Minnetronix Inc., and Voyager Therapeutics.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

