Parasite associations predict infection risk: incorporating co-infections in predictive models for neglected tropical diseases
- PMID: 32178706
- PMCID: PMC7077138
- DOI: 10.1186/s13071-020-04016-2
Parasite associations predict infection risk: incorporating co-infections in predictive models for neglected tropical diseases
Abstract
Background: Schistosomiasis and infection by soil-transmitted helminths are some of the world's most prevalent neglected tropical diseases. Infection by more than one parasite (co-infection) is common and can contribute to clinical morbidity in children. Geostatistical analyses of parasite infection data are key for developing mass drug administration strategies, yet most methods ignore co-infections when estimating risk. Infection status for multiple parasites can act as a useful proxy for data-poor individual-level or environmental risk factors while avoiding regression dilution bias. Conditional random fields (CRF) is a multivariate graphical network method that opens new doors in parasite risk mapping by (i) predicting co-infections with high accuracy; (ii) isolating associations among parasites; and (iii) quantifying how these associations change across landscapes.
Methods: We built a spatial CRF to estimate infection risks for Ascaris lumbricoides, Trichuris trichiura, hookworms (Ancylostoma duodenale and Necator americanus) and Schistosoma mansoni using data from a national survey of Rwandan schoolchildren. We used an ensemble learning approach to generate spatial predictions by simulating from the CRF's posterior distribution with a multivariate boosted regression tree that captured non-linear relationships between predictors and covariance in infection risks. This CRF ensemble was compared against single parasite gradient boosted machines to assess each model's performance and prediction uncertainty.
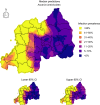
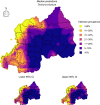
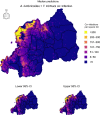
Results: Parasite co-infections were common, with 19.57% of children infected with at least two parasites. The CRF ensemble achieved higher predictive power than single-parasite models by improving estimates of co-infection prevalence at the individual level and classifying schools into World Health Organization treatment categories with greater accuracy. The CRF uncovered important environmental and demographic predictors of parasite infection probabilities. Yet even after capturing demographic and environmental risk factors, the presences or absences of other parasites were strong predictors of individual-level infection risk. Spatial predictions delineated high-risk regions in need of anthelminthic treatment interventions, including areas with higher than expected co-infection prevalence.
Conclusions: Monitoring studies routinely screen for multiple parasites, yet statistical models generally ignore this multivariate data when assessing risk factors and designing treatment guidelines. Multivariate approaches can be instrumental in the global effort to reduce and eventually eliminate neglected helminth infections in developing countries.
Keywords: Conditional random fields; Neglected tropical disease; Parasite co-infection; Schistosoma mansoni; Soil-transmitted helminths; Spatial epidemiology.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
National mapping of soil-transmitted helminth and schistosome infections in Ethiopia.Parasit Vectors. 2020 Sep 1;13(1):437. doi: 10.1186/s13071-020-04317-6. Parasit Vectors. 2020. PMID: 32873333 Free PMC article.
-
Spatial distribution and populations at risk of A. lumbricoides and T. trichiura co-infections and infection intensity classes: an ecological study.Parasit Vectors. 2018 Oct 3;11(1):535. doi: 10.1186/s13071-018-3107-y. Parasit Vectors. 2018. PMID: 30285906 Free PMC article.
-
Effect of anthelminthic treatment on helminth infection and related anaemia among school-age children in northwestern Ethiopia.BMC Infect Dis. 2016 Oct 28;16(1):613. doi: 10.1186/s12879-016-1956-6. BMC Infect Dis. 2016. PMID: 27793110 Free PMC article.
-
Geohelminths: public health significance.J Infect Dev Ctries. 2014 Jan 15;8(1):5-16. doi: 10.3855/jidc.3183. J Infect Dev Ctries. 2014. PMID: 24423707 Review.
-
Intestinal parasites co-infection among tuberculosis patients in Ethiopia: a systematic review and meta-analysis.BMC Infect Dis. 2020 Jul 14;20(1):510. doi: 10.1186/s12879-020-05237-7. BMC Infect Dis. 2020. PMID: 32664873 Free PMC article.
Cited by
-
Coinfection by multiple Trypanosoma cruzi clones: a new perspective on host-parasite relationship with consequences for pathogenesis and management of Chagas disease.Microbiol Mol Biol Rev. 2025 Jun 25;89(2):e0024224. doi: 10.1128/mmbr.00242-24. Epub 2025 Mar 21. Microbiol Mol Biol Rev. 2025. PMID: 40116484 Review.
-
Environmental, anthropogenic, and spatial factors affecting species composition and species associations in helminth communities of water frogs (Pelophylax esculentus complex) in Latvia.Parasitol Res. 2021 Oct;120(10):3461-3474. doi: 10.1007/s00436-021-07303-8. Epub 2021 Sep 3. Parasitol Res. 2021. PMID: 34476585
-
Grand challenges in parasite epidemiology and ecology.Front Parasitol. 2022 Oct 3;1:1034819. doi: 10.3389/fpara.2022.1034819. eCollection 2022. Front Parasitol. 2022. PMID: 39816463 Free PMC article. No abstract available.
-
Household profiles of neglected tropical disease symptoms among children: A latent class analysis of built-environment features of Tanzanian households using the Demographic and Health Survey.J Glob Health. 2022 Sep 3;12:04067. doi: 10.7189/jogh.12.04067. J Glob Health. 2022. PMID: 36057837 Free PMC article.
-
Evolutionary consequences of vector-borne transmission: how using vectors shapes host, vector and pathogen evolution.Parasitology. 2022 Nov;149(13):1667-1678. doi: 10.1017/S0031182022001378. Epub 2022 Oct 6. Parasitology. 2022. PMID: 36200511 Free PMC article. Review.
References
-
- Ortu G, Assoum M, Wittmann U, Knowles S, Clements M, Ndayishimiye O, et al. The impact of an 8-year mass drug administration programme on prevalence, intensity and co-infections of soil-transmitted helminthiases in Burundi. Parasit Vectors. 2016;9:513. doi: 10.1186/s13071-016-1794-9. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

