End-stage renal disease is different from chronic kidney disease in upregulating ROS-modulated proinflammatory secretome in PBMCs - A novel multiple-hit model for disease progression
- PMID: 32179051
- PMCID: PMC7327976
- DOI: 10.1016/j.redox.2020.101460
End-stage renal disease is different from chronic kidney disease in upregulating ROS-modulated proinflammatory secretome in PBMCs - A novel multiple-hit model for disease progression
Abstract
Background: The molecular mechanisms underlying chronic kidney disease (CKD) transition to end-stage renal disease (ESRD) and CKD acceleration of cardiovascular and other tissue inflammations remain poorly determined.
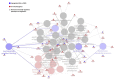
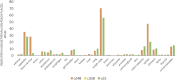
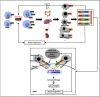
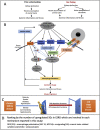
Methods: We conducted a comprehensive data analyses on 7 microarray datasets in peripheral blood mononuclear cells (PBMCs) from patients with CKD and ESRD from NCBI-GEO databases, where we examined the expressions of 2641 secretome genes (SG).
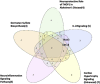
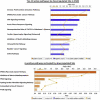
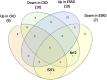
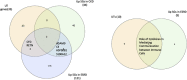
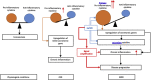
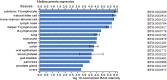
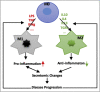
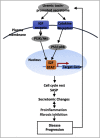
Results: 1) 86.7% middle class (molecular weight >500 Daltons) uremic toxins (UTs) were encoded by SGs; 2) Upregulation of SGs in PBMCs in patients with ESRD (121 SGs) were significantly higher than that of CKD (44 SGs); 3) Transcriptomic analyses of PBMC secretome had advantages to identify more comprehensive secretome than conventional secretomic analyses; 4) ESRD-induced SGs had strong proinflammatory pathways; 5) Proinflammatory cytokines-based UTs such as IL-1β and IL-18 promoted ESRD modulation of SGs; 6) ESRD-upregulated co-stimulation receptors CD48 and CD58 increased secretomic upregulation in the PBMCs, which were magnified enormously in tissues; 7) M1-, and M2-macrophage polarization signals contributed to ESRD- and CKD-upregulated SGs; 8) ESRD- and CKD-upregulated SGs contained senescence-promoting regulators by upregulating proinflammatory IGFBP7 and downregulating anti-inflammatory TGF-β1 and telomere stabilizer SERPINE1/PAI-1; 9) ROS pathways played bigger roles in mediating ESRD-upregulated SGs (11.6%) than that in CKD-upregulated SGs (6.8%), and half of ESRD-upregulated SGs were ROS-independent.
Conclusions: Our analysis suggests novel secretomic upregulation in PBMCs of patients with CKD and ESRD, act synergistically with uremic toxins, to promote inflammation and potential disease progression. Our findings have provided novel insights on PBMC secretome upregulation to promote disease progression and may lead to the identification of new therapeutic targets for novel regimens for CKD, ESRD and their accelerated cardiovascular disease, other inflammations and cancers. (Total words: 279).
Keywords: (CKD); (ESRD); (ROS); Chronic kidney disease; End-stage renal disease; PBMC secretome; Reactive oxygen species; Trained immunity.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Centers for Disease Control and Prevention . US Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2019. Chronic Kidney Disease in the United States, 2019.https://www.cdc.gov/kidneydisease/publications-resources/2019-national-f...
-
- Reikes S.T. Trends in end-stage renal disease. Epidemiology, morbidity, and mortality. PGM (Postgrad. Med.) 2000;108(1):124–126. 9-31, 35-6 passim. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

