Antinociceptive and Immune Effects of Delta-9-Tetrahydrocannabinol or Cannabidiol in Male Versus Female Rats with Persistent Inflammatory Pain
- PMID: 32179573
- PMCID: PMC7250366
- DOI: 10.1124/jpet.119.263319
Antinociceptive and Immune Effects of Delta-9-Tetrahydrocannabinol or Cannabidiol in Male Versus Female Rats with Persistent Inflammatory Pain
Abstract
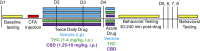
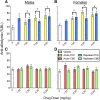
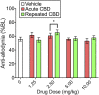
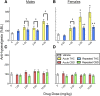
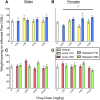
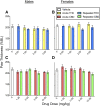
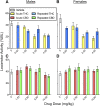
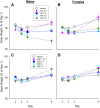
Chronic pain is the most common reason reported for using medical cannabis. The goal of this research was to determine whether the two primary phytocannabinoids, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), are effective treatments for persistent inflammatory pain. In experiment 1, inflammation was induced by intraplantar injection of Complete Freund's adjuvant (CFA). Then THC (0.0-4.0 mg/kg, i.p.) or CBD (0.0-10 mg/kg, i.p.) was administered twice daily for 3 days. On day 4, THC, CBD, or vehicle was administered, and allodynia, hyperalgesia, weight-bearing, locomotor activity, and hindpaw edema were assessed 0.5-4 hours postinjection. In experiment 2, CFA or mineral oil (no-pain control)-treated rats were given THC (2.0 mg/kg, i.p.), CBD (10 mg/kg, i.p.), or vehicle in the same manner as in experiment 1. Four hours postinjection on day 4, serum samples were taken for analysis of cytokines known to influence inflammatory pain: interleukin (IL)-1β, IL-6, IL-10, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α THC dose-dependently reduced pain-related behaviors but did not reduce hindpaw edema, and little tolerance developed to THC's effects. In contrast, CBD effects on inflammatory pain were minimal. THC produced little to no change in serum cytokines, whereas CBD decreased IL-1β, IL-10, and IFN-γ and increased IL-6. Few sex differences in antinociception or immune modulation were observed with either drug, but CFA-induced immune activation was significantly greater in males than females. These results suggest that THC may be more beneficial than CBD for reducing inflammatory pain in that THC maintains its efficacy with short-term treatment in both sexes and does not induce immune activation. SIGNIFICANCE STATEMENT: The pain-relieving effects of cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC) are examined in male and female rats with persistent inflammatory pain to determine whether individual phytocannabinoids could be a viable treatment for men and women with chronic inflammatory pain. Additionally, sex differences in the immune response to an adjuvant and to THC and CBD are characterized to provide preliminary insight into immune-related effects of cannabinoid-based therapy for pain.
Copyright © 2020 by The American Society for Pharmacology and Experimental Therapeutics.
Figures
References
-
- Bass CE, Martin BR. (2000) Time course for the induction and maintenance of tolerance to Δ(9)-tetrahydrocannabinol in mice. Drug Alcohol Depend 60:113–119. - PubMed
-
- Blyth FM, March LM, Brnabic AJM, Jorm LR, Williamson M, Cousins MJ. (2001) Chronic pain in Australia: a prevalence study. Pain 89:127–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

