Identification of FMR1-regulated molecular networks in human neurodevelopment
- PMID: 32179589
- PMCID: PMC7111522
- DOI: 10.1101/gr.251405.119
Identification of FMR1-regulated molecular networks in human neurodevelopment
Abstract
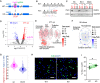
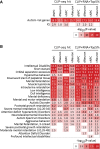
RNA-binding proteins (RNA-BPs) play critical roles in development and disease to regulate gene expression. However, genome-wide identification of their targets in primary human cells has been challenging. Here, we applied a modified CLIP-seq strategy to identify genome-wide targets of the FMRP translational regulator 1 (FMR1), a brain-enriched RNA-BP, whose deficiency leads to Fragile X Syndrome (FXS), the most prevalent inherited intellectual disability. We identified FMR1 targets in human dorsal and ventral forebrain neural progenitors and excitatory and inhibitory neurons differentiated from human pluripotent stem cells. In parallel, we measured the transcriptomes of the same four cell types upon FMR1 gene deletion. We discovered that FMR1 preferentially binds long transcripts in human neural cells. FMR1 targets include genes unique to human neural cells and associated with clinical phenotypes of FXS and autism. Integrative network analysis using graph diffusion and multitask clustering of FMR1 CLIP-seq and transcriptional targets reveals critical pathways regulated by FMR1 in human neural development. Our results demonstrate that FMR1 regulates a common set of targets among different neural cell types but also operates in a cell type-specific manner targeting distinct sets of genes in human excitatory and inhibitory neural progenitors and neurons. By defining molecular subnetworks and validating specific high-priority genes, we identify novel components of the FMR1 regulation program. Our results provide new insights into gene regulation by a critical neuronal RNA-BP in human neurodevelopment.
© 2020 Li et al.; Published by Cold Spring Harbor Laboratory Press.
Figures




References
-
- Avitzour M, Mor-Shaked H, Yanovsky-Dagan S, Aharoni S, Altarescu G, Renbaum P, Eldar-Geva T, Schonberger O, Levy-Lahad E, Epsztejn-Litman S, et al. 2014. FMR1 epigenetic silencing commonly occurs in undifferentiated fragile X-affected embryonic stem cells. Stem Cell Reports 3: 699–706. 10.1016/j.stemcr.2014.09.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 HD085288/HD/NICHD NIH HHS/United States
- R01 NS105200/NS/NINDS NIH HHS/United States
- R56 MH113146/MH/NIMH NIH HHS/United States
- R03 HD083538/HD/NICHD NIH HHS/United States
- R01 MH118827/MH/NIMH NIH HHS/United States
- R01 MH116582/MH/NIMH NIH HHS/United States
- R21 NS105339/NS/NINDS NIH HHS/United States
- R01 GM117339/GM/NIGMS NIH HHS/United States
- R21 NS098767/NS/NINDS NIH HHS/United States
- R01 AG053987/AG/NIA NIH HHS/United States
- U54 HD090256/HD/NICHD NIH HHS/United States
- R01 MH078972/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
