Scaling up single-cell mechanics to multicellular tissues - the role of the intermediate filament-desmosome network
- PMID: 32179593
- PMCID: PMC7097224
- DOI: 10.1242/jcs.228031
Scaling up single-cell mechanics to multicellular tissues - the role of the intermediate filament-desmosome network
Abstract
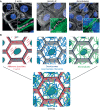
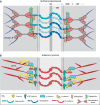
Cells and tissues sense, respond to and translate mechanical forces into biochemical signals through mechanotransduction, which governs individual cell responses that drive gene expression, metabolic pathways and cell motility, and determines how cells work together in tissues. Mechanotransduction often depends on cytoskeletal networks and their attachment sites that physically couple cells to each other and to the extracellular matrix. One way that cells associate with each other is through Ca2+-dependent adhesion molecules called cadherins, which mediate cell-cell interactions through adherens junctions, thereby anchoring and organizing the cortical actin cytoskeleton. This actin-based network confers dynamic properties to cell sheets and developing organisms. However, these contractile networks do not work alone but in concert with other cytoarchitectural elements, including a diverse network of intermediate filaments. This Review takes a close look at the intermediate filament network and its associated intercellular junctions, desmosomes. We provide evidence that this system not only ensures tissue integrity, but also cooperates with other networks to create more complex tissues with emerging properties in sensing and responding to increasingly stressful environments. We will also draw attention to how defects in intermediate filament and desmosome networks result in both chronic and acquired diseases.
Keywords: Cadherin; Cell–cell adhesion; Cytoskeleton; Desmosome; Intermediate filaments; Mechanotransduction.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Baddam S. R., Arsenovic P. T., Narayanan V., Duggan N. R., Mayer C. R., Newman S. T., Abutaleb D. A., Mohan A., Kowalczyk A. P. and Conway D. E. (2018). The desmosomal cadherin desmoglein-2 experiences mechanical tension as demonstrated by a FRET-based tension biosensor expressed in living cells. Cells 7, E66 10.3390/cells7070066 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

