WRKY13 Enhances Cadmium Tolerance by Promoting D-CYSTEINE DESULFHYDRASE and Hydrogen Sulfide Production
- PMID: 32179630
- PMCID: PMC7210638
- DOI: 10.1104/pp.19.01504
WRKY13 Enhances Cadmium Tolerance by Promoting D-CYSTEINE DESULFHYDRASE and Hydrogen Sulfide Production
Abstract
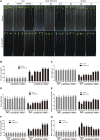
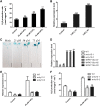
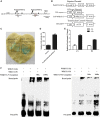
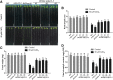
Hydrogen sulfide (H2S), a plant gasotransmitter, functions in the plant response to cadmium (Cd) stress, implying a role for cysteine desulfhydrase in producing H2S in this process. Whether d -CYSTEINE DESULFHYDRASE (DCD) acts in the plant Cd response remains to be identified, and if it does, how DCD is regulated in this process is also unknown. Here, we report that DCD-mediated H2S production enhances plant Cd tolerance in Arabidopsis (Arabidopsis thaliana). When subjected to Cd stress, a dcd mutant accumulated more Cd and reactive oxygen species and showed increased Cd sensitivity, whereas transgenic lines overexpressing DCD had decreased Cd and reactive oxygen species levels and were more tolerant to Cd stress compared with wild-type plants. Furthermore, the expression of DCD was stimulated by Cd stress, and this up-regulation was mediated by a Cd-induced transcription factor, WRKY13, which bound to the DCD promoter. Consistently, the higher Cd sensitivity of the wrky13-3 mutant was rescued by the overexpression of DCD Together, our results demonstrate that Cd-induced WRKY13 activates DCD expression to increase the production of H2S, leading to higher Cd tolerance in plants.
© 2020 American Society of Plant Biologists. All Rights Reserved.
Figures



Similar articles
-
Hydrogen sulfide - cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots.Sci Rep. 2016 Dec 22;6:39702. doi: 10.1038/srep39702. Sci Rep. 2016. PMID: 28004782 Free PMC article.
-
Hydrogen sulfide generated by L-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure.Plant Physiol. 2014 Dec;166(4):2065-76. doi: 10.1104/pp.114.245373. Epub 2014 Sep 29. Plant Physiol. 2014. PMID: 25266633 Free PMC article.
-
L-Cysteine desulfhydrase-dependent hydrogen sulfide is required for methane-induced lateral root formation.Plant Mol Biol. 2019 Feb;99(3):283-298. doi: 10.1007/s11103-018-00817-3. Epub 2019 Jan 8. Plant Mol Biol. 2019. PMID: 30623274
-
Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants - from the field to the test tube and back.Plant Biol (Stuttg). 2007 Sep;9(5):582-8. doi: 10.1055/s-2007-965424. Plant Biol (Stuttg). 2007. PMID: 17853358 Review.
-
Regulation of cystathionine gamma-lyase/H₂S system and its pathological implication.Front Biosci (Landmark Ed). 2014 Jun 1;19(8):1355-69. doi: 10.2741/4286. Front Biosci (Landmark Ed). 2014. PMID: 24896355 Review.
Cited by
-
A D-cysteine desulfhydrase, SlDCD2, participates in tomato fruit ripening by modulating ROS homoeostasis and ethylene biosynthesis.Hortic Res. 2023 Feb 1;10(3):uhad014. doi: 10.1093/hr/uhad014. eCollection 2023 Mar. Hortic Res. 2023. PMID: 36968183 Free PMC article.
-
Genome-wide analysis of WRKY gene family and the dynamic responses of key WRKY genes involved in cadmium stress in Brassica juncea.Front Plant Sci. 2024 Oct 10;15:1465905. doi: 10.3389/fpls.2024.1465905. eCollection 2024. Front Plant Sci. 2024. PMID: 39450073 Free PMC article.
-
A methylation that offers plants protection.Plant Physiol. 2022 Nov 28;190(4):2082-2084. doi: 10.1093/plphys/kiac458. Plant Physiol. 2022. PMID: 36149313 Free PMC article. No abstract available.
-
Leveraging multi-omics tools to comprehend responses and tolerance mechanisms of heavy metals in crop plants.Funct Integr Genomics. 2024 Oct 23;24(6):194. doi: 10.1007/s10142-024-01481-1. Funct Integr Genomics. 2024. PMID: 39441418 Review.
-
RsWRKY75 promotes ROS scavenging and cadmium efflux via activating the transcription of RsAPX1 and RsPDR8 in radish (Raphanus sativus L.).Plant Cell Rep. 2025 Feb 24;44(3):65. doi: 10.1007/s00299-025-03445-6. Plant Cell Rep. 2025. PMID: 39994016
References
-
- Ahsan N, Nakamura T, Komatsu S(2012) Differential responses of microsomal proteins and metabolites in two contrasting cadmium (Cd)-accumulating soybean cultivars under Cd stress. Amino Acids 42: 317–327 - PubMed
-
- Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM(2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57: 233–266 - PubMed
-
- Brückner H, Westhauser T(2003) Chromatographic determination of L- and D-amino acids in plants. Amino Acids 24: 43–55 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

