A first-in-human phase 1 dose escalation study of spartalizumab (PDR001), an anti-PD-1 antibody, in patients with advanced solid tumors
- PMID: 32179633
- PMCID: PMC7073791
- DOI: 10.1136/jitc-2020-000530
A first-in-human phase 1 dose escalation study of spartalizumab (PDR001), an anti-PD-1 antibody, in patients with advanced solid tumors
Abstract
Background: Spartalizumab is a humanized IgG4κ monoclonal antibody that binds programmed death-1 (PD-1) and blocks its interaction with PD-L1 and PD-L2. This phase 1/2 study was designed to assess the safety, pharmacokinetics, and preliminary efficacy of spartalizumab in patients with advanced or metastatic solid tumors.
Methods: In the phase 1 part of the study, 58 patients received spartalizumab, intravenously, at doses of 1, 3, or 10 mg/kg, administered every 2 weeks (Q2W), or 3 or 5 mg/kg every 4 weeks (Q4W).
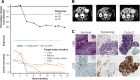
Results: Patients had a wide range of tumor types, most commonly sarcoma (28%) and metastatic renal cell carcinoma (10%); other tumor types were reported in ≤3 patients each. Most patients (93%) had received prior antineoplastic therapy (median three prior lines) and two-thirds of the population had tumor biopsies negative for PD-L1 expression at baseline. The maximum tolerated dose was not reached. The recommended phase 2 doses were selected as 400 mg Q4W or 300 mg Q3W. No dose-limiting toxicities were observed, and adverse events included those typical of other PD-1 antibodies. The most common treatment-related adverse events of any grade were fatigue (22%), diarrhea (17%), pruritus (14%), hypothyroidism (10%), and nausea (10%). Partial responses occurred in two patients (response rate 3.4%); one with atypical carcinoid tumor of the lung and one with anal cancer. Paired tumor biopsies from patients taken at baseline and on treatment suggested an on-treatment increase in CD8+ lymphocyte infiltration in patients with clinical benefit.
Conclusions: Spartalizumab was well tolerated at all doses tested in patients with previously treated advanced solid tumors. On-treatment immune activation was seen in tumor biopsies; however, limited clinical activity was reported in this heavily pretreated, heterogeneous population. The phase 2 part of this study is ongoing in select tumor types.
Trial registration number: NCT02404441.
Keywords: clinical trials as topic; immunotherapy; programmed cell death 1 receptor.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AN received research funding from NCI, EMD Serono, MedImmune, Healios Oncology Nutrition, Atterocor, Amplimmune, ARMO BioSciences, Eli Lilly, Karyopharm Therapeutics, Incyte, Regeneron, Merck, BMS, Pfizer, Neon Therapeutics, Calithera Biosciences, TopAlliance Biosciences, Kymab, PsiOxus, and Immune Deficiency Foundation (spouse); travel/accommodation expense from ARMO BioSciences; and consultancy fees and research funding from CytomX Therapeutics and Novartis. JG received grant funding from Array, Tesaro, Moderna, Adaptimmune, and Alexo; personal fees from Oncorus, Regeneron, Pfizer, Incyte, Agios, Amgen, and Ironwood Pharmaceuticals; and grant funding and personal fees from Bristol-Myers Squibb, Genentech/Roche, Takeda, Blueprint, Loxo, Novartis, and Merck. PMF received research funding from Corvus and Kyowa; consultancy fees from AbbVie, Boehringer, EMD Serono, Inivata, Janssen, Lilly, and Merck; and research funding and consultancy fees from AstraZeneca, BMS, and Novartis. MOB received funding from Novartis, personal fees from BMS, Adaptimmune, GSK, Novartis, EMD Serono, Sanofi, Immunocore, and Turnstone; grant funding from Takara Bio; and personal fees and grant funding from Merck. C-CL received personal fees from BeiGene, Daiichi Sankyo, Roche, and Novartis. SS received research funding from GSK, Millennium, MedImmune, Johnson & Johnson, Gilead, Plexxikon, Onyx, Bayer, Blueprint, XuanZhu, Incyte, Toray, Celgene, Hengrui, OncoMed, Tesaro, AADi, Merck, Inhibrx, AMAL, and Syndax; personal fees and research funding from Novartis; equity from Iterion Therapeutics, Proterus Therapeutics, ConverGene, and Stingray Therapeutics; honoraria from Exelixis, Loxo Oncology, Natera, Hengrui Therapeutics, Tarveda Therapeutics, Dracen Pharmaceuticals, and Barricade Therapeutics; and owns stock with LSK BioPharma and Salarius Pharmaceuticals. MT received consultancy fees from Eisai, Bristol-Myers Squibb, Array Biopharma, Blueprint Medicines, Arqule, Loxo Oncology, Bayer, Novartis, and Genentech. JHMS received personal fees from Modra Pharmaceuticals. TMB received grant funding from Daiichi Sankyo, Medpacto, Incyte, Mirati Therapeutics, MedImmune, AbbVie, AstraZeneca, MabVax, Stemline Therapeutics, Merck, Lilly, GlaxoSmithKline, Novartis, Genentech, Deciphera, Merrimack, Immunogen, Millennium, Phosplatin Therapeutics, Calithera Biosciences, Kolltan Pharmaceuticals, Principa Biopharma, Peleton, Immunocore, Roche, Aileron Therapeutics, Bristol-Myers Squibb, Amgen, Onyx, Sanofi, Boehringer-Ingelheim, Astellas Pharma, Five Prime Therapeutics, Jacobio, Top Alliance BioScience, Janssen, Clovis Oncology, Takeda, Karyopharm Therapeutics, Foundation Medicine, and ARMO Biosciences; grant funding and consultancy fees from Leap Therapeutics; grant funding, consultancy fees, and non-financial support from Ignyta and Moderna Therapeutics; grant funding, personal fees, and consultancy fees from Pfizer; grant funding, personal fees, and non-financial support from Loxo and Bayer; personal fees and non-financial support from Guardant Health; and personal fees from Exelesis. HW, HS, and JM are employees of Novartis. APS and JF are employees of Novartis and own stock with Novartis. SC is an employee of Novartis and has patents with Novartis.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
