Oxaliplatin Pt(IV) prodrugs conjugated to gadolinium-texaphyrin as potential antitumor agents
- PMID: 32179677
- PMCID: PMC7132275
- DOI: 10.1073/pnas.1914911117
Oxaliplatin Pt(IV) prodrugs conjugated to gadolinium-texaphyrin as potential antitumor agents
Abstract
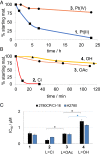
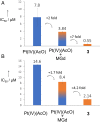
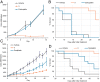
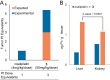
Described here is the development of gadolinium(III) texaphyrin-platinum(IV) conjugates capable of overcoming platinum resistance by 1) localizing to solid tumors, 2) promoting enhanced cancer cell uptake, and 3) reactivating p53 in platinum-resistant models. Side by side comparative studies of these Pt(IV) conjugates to clinically approved platinum(II) agents and previously reported platinum(II)-texaphyrin conjugates demonstrate that the present Pt(IV) conjugates are more stable against hydrolysis and nucleophilic attack. Moreover, they display high potent antiproliferative activity in vitro against human and mouse cell cancer lines. Relative to the current platinum clinical standard of care (SOC), a lead Gd(III) texaphyrin-Pt(IV) prodrug conjugate emerging from this development effort was found to be more efficacious in subcutaneous (s.c.) mouse models involving both cell-derived xenografts and platinum-resistant patient-derived xenografts. Comparative pathology studies in mice treated with equimolar doses of the lead Gd texaphyrin-Pt(IV) conjugate or the US Food and Drug Administration (FDA)-approved agent oxaliplatin revealed that the conjugate was better tolerated. Specifically, the lead could be dosed at more than three times (i.e., 70 mg/kg per dose) the tolerable dose of oxaliplatin (i.e., 4 to 6 mg/kg per dose depending on the animal model) with little to no observable adverse effects. A combination of tumor localization, redox cycling, and reversible protein binding is invoked to explain the relatively increased tolerability and enhanced anticancer activity seen in vivo. On the basis of the present studies, we conclude that metallotexaphyrin-Pt conjugates may have substantial clinical potential as antitumor agents.
Keywords: cancer; drug development; drug resistance; platinum prodrug; texaphyrins.
Conflict of interest statement
Competing interest statement: Since the time of the original submission, the texaphyrin conjugates described in this manuscript were licensed by The University of Texas to the IQ Global Group and planned for further development by a new for-profit company, OncoTEX Inc. J.F.A. is now employed by OncoTEX Inc., and J.L.S. now serves as a nonexecutive board member for OncoTEX Inc. The other authors declare no conflict of interest.
Figures




References
-
- Rosenberg B., VanCamp L., Trosko J. E., Mansour V. H., Platinum compounds: A new class of potent antitumour agents. Nature 222, 385–386 (1969). - PubMed
-
- Galanski M., Jakupec M. A., Keppler B. K., Update of the preclinical situation of anticancer platinum complexes: Novel design strategies and innovative analytical approaches. Curr. Med. Chem. 12, 2075–2094 (2005). - PubMed
-
- Ozols R. F., Challenges for chemotherapy in ovarian cancer. Ann. Oncol. 17 (suppl. 5), v181–v187 (2006). - PubMed
-
- Siddik Z. H., Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 22, 7265–7279 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

