Targeting inhibitory cerebellar circuitry to alleviate behavioral deficits in a mouse model for studying idiopathic autism
- PMID: 32179875
- PMCID: PMC7234983
- DOI: 10.1038/s41386-020-0656-5
Targeting inhibitory cerebellar circuitry to alleviate behavioral deficits in a mouse model for studying idiopathic autism
Abstract
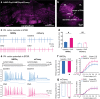
Autism spectrum disorder (ASD) encompasses wide-ranging neuropsychiatric symptoms with unclear etiology. Although the cerebellum is a key region implicated in ASD, it remains elusive how the cerebellar circuitry is altered and whether the cerebellum can serve as a therapeutic target to rectify the phenotype of idiopathic ASD with polygenic abnormalities. Using a syndromic ASD model, e.g., Black and Tan BRachyury T+Itpr3tf/J (BTBR) mice, we revealed that increased excitability of presynaptic interneurons (INs) and decreased intrinsic excitability of postsynaptic Purkinje neurons (PNs) resulted in low PN firing rates in the cerebellum. Knowing that downregulation of Kv1.2 potassium channel in the IN nerve terminals likely augmented their excitability and GABA release, we applied a positive Kv1.2 modulator to mitigate the presynaptic over-inhibition and social impairment of BTBR mice. Selective restoration of the PN activity by a new chemogenetic approach alleviated core ASD-like behaviors of the BTBR strain. These findings highlight complex mechanisms converging onto the cerebellar dysfunction in the phenotypic model and provide effective strategies for potential therapies of ASD.
Figures




References
-
- Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383:896–910. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

