Uric Acid and Hypertension: An Update With Recommendations
- PMID: 32179896
- PMCID: PMC7368167
- DOI: 10.1093/ajh/hpaa044
Uric Acid and Hypertension: An Update With Recommendations
Erratum in
-
Corrigendum to: Uric Acid and Hypertension: An Update with Recommendations.Am J Hypertens. 2020 Dec 31;33(12):1150. doi: 10.1093/ajh/hpaa118. Am J Hypertens. 2020. PMID: 32935830 Free PMC article. No abstract available.
Abstract
The association between increased serum urate and hypertension has been a subject of intense controversy. Extracellular uric acid drives uric acid deposition in gout, kidney stones, and possibly vascular calcification. Mendelian randomization studies, however, indicate that serum urate is likely not the causal factor in hypertension although it does increase the risk for sudden cardiac death and diabetic vascular disease. Nevertheless, experimental evidence strongly suggests that an increase in intracellular urate is a key factor in the pathogenesis of primary hypertension. Pilot clinical trials show beneficial effect of lowering serum urate in hyperuricemic individuals who are young, hypertensive, and have preserved kidney function. Some evidence suggest that activation of the renin-angiotensin system (RAS) occurs in hyperuricemia and blocking the RAS may mimic the effects of xanthine oxidase inhibitors. A reduction in intracellular urate may be achieved by lowering serum urate concentration or by suppressing intracellular urate production with dietary measures that include reducing sugar, fructose, and salt intake. We suggest that these elements in the western diet may play a major role in the pathogenesis of primary hypertension. Studies are necessary to better define the interrelation between uric acid concentrations inside and outside the cell. In addition, large-scale clinical trials are needed to determine if extracellular and intracellular urate reduction can provide benefit hypertension and cardiometabolic disease.
Keywords: blood pressure; fructose; hypertension; renin–angiotensin system; uric acid; xanthine oxidase.
© American Journal of Hypertension, Ltd 2020. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


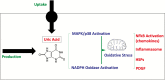
References
-
- Mahomed FA. On chronic Bright’s disease, and its essential symptoms. Lancet 1879; I:398–404.
-
- Johnson RJ, Bakris GL, Borghi C, Chonchol MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB, Sanchez Lozada LG, Stahl E, Weiner DE, Chertow GM. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis 2018; 71: 851– 865. - PMC - PubMed
-
- Johnson RJ, Nakagawa T, Sánchez-Lozada LG, Lanaspa MA, Tamura Y, Tanabe K, Ishimoto T, Thomas J, Inaba S, Kitagawa W, Rivard CJ. Umami: the taste that drives purine intake. J Rheumatol 2013; 40:1794–1796. - PubMed
-
- Lanaspa MA, Ishimoto T, Li N, Cicerchi C, Orlicky DJ, Ruzycki P, Ruzicky P, Rivard C, Inaba S, Roncal-Jimenez CA, Bales ES, Diggle CP, Asipu A, Petrash JM, Kosugi T, Maruyama S, Sanchez-Lozada LG, McManaman JL, Bonthron DT, Sautin YY, Johnson RJ. Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat Commun 2013; 4:2434. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

