Utilization of Direct Oral Anticoagulants in People Living with Human Immunodeficiency Virus: Observational Data from the District of Columbia Cohort
- PMID: 32179901
- PMCID: PMC7744993
- DOI: 10.1093/cid/ciaa284
Utilization of Direct Oral Anticoagulants in People Living with Human Immunodeficiency Virus: Observational Data from the District of Columbia Cohort
Abstract
Background: Direct oral anticoagulants (DOACs) have become first-line treatment for venous thrombotic events. DOAC prescribing trends among people living with human immunodeficiency virus (PWH) are not well described. The coadministration of DOACs with the antiretroviral (ARV) pharmacokinetic boosters ritonavir (RTV) or cobicistat (COBI) may be complicated by pharmacokinetic interactions.
Methods: A longitudinal cohort study was conducted using the D.C. Cohort Database in Washington, D.C., from January 2011 to March 2017, to describe oral anticoagulant prescribing among PWH ≥ 18 years old and the prevalence of DOAC use with RTV or COBI. Data collection included demographic and clinical characteristics, ARV and anticoagulant prescriptions, and International Classification of Diseases Ninth and Tenth Edition diagnosis codes.
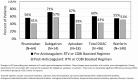
Results: Among 8315 PWH, there were 236 anticoagulant prescriptions (96 DOAC, 140 warfarin) for 206 persons. PWH prescribed anticoagulants were predominantly Black (82%) and male (82%), with a mean age at anticoagulant initiation of 56 years. DOAC use increased from 3% of total anticoagulant prescribing in 2011 to 43% in 2016, accounting for 64% of all newly recorded anticoagulant prescriptions by 2016. There were 19 bleeding events recorded among 16 individuals. Despite the Food and Drug Administration label recommendation to avoid rivaroxaban with boosted ARVs, 41% remained on boosted ARVs after rivaroxaban initiation.
Conclusions: DOAC use increased substantially in PWH by 2016. Although rivaroxaban is not recommended with RTV or COBI, concomitant use was recorded in 41% of rivaroxaban recipients in this cohort. As DOAC usage increases, clinicians need to be aware of potential DOAC/ARV interactions in order to select the most appropriate oral anticoagulant and monitoring plan for PWH.
Keywords: HIV; anticoagulation; antiretrovirals; cobicistat; ritonavir.
Published by Oxford University Press for the Infectious Diseases Society of America 2020.
Figures

References
-
- Kearon C, Akl EA, Ornelas J, et al. . Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016; 149:315–52. - PubMed
-
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol 2014; 64(21): 2246–2280, - PubMed
-
- Institute for Safe Medication Practices. Quarter watch – 2016 quarter four. Available at: https://www.ismp.org/quarterwatch/annual-report-2016. Accessed on 29 July 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

