Enzymatic studies on aromatic prenyltransferases
- PMID: 32180104
- PMCID: PMC7253389
- DOI: 10.1007/s11418-020-01393-x
Enzymatic studies on aromatic prenyltransferases
Erratum in
-
Correction to: Enzymatic studies on aromatic prenyltransferases.J Nat Med. 2020 Sep;74(4):840. doi: 10.1007/s11418-020-01415-8. J Nat Med. 2020. PMID: 32468288 Free PMC article.
Abstract
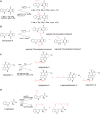
Aromatic prenyltransferases (PTases), including ABBA-type and dimethylallyl tryptophan synthase (DMATS)-type enzymes from bacteria and fungi, play important role for diversification of the natural products and improvement of the biological activities. For a decade, the characterization of enzymes and enzymatic synthesis of prenylated compounds by using ABBA-type and DMATS-type PTases have been demonstrated. Here, I introduce several examples of the studies on chemoenzymatic synthesis of unnatural prenylated compounds and the enzyme engineering of ABBA-type and DMATS-type PTases.
Keywords: Aromatic prenyltransferase; Biosynthesis; Enzyme engineering; Prenylated compounds.
Figures
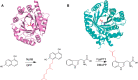



References
-
- Alhassan AM, Abdullahi MI, Uba A, Umar A. Prenylation of aromatic secondary metabolites: a new frontier for development of novel drugs. Trop J Pharm Res. 2014;13:307–314. doi: 10.4314/tjpr.v13i2.22. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

