Postnatal corticosteroids for transient tachypnoea of the newborn
- PMID: 32180216
- PMCID: PMC7076329
- DOI: 10.1002/14651858.CD013222.pub2
Postnatal corticosteroids for transient tachypnoea of the newborn
Abstract
Background: Transient tachypnoea of the newborn (TTN) is characterized by tachypnoea and signs of respiratory distress. Transient tachypnoea typically appears within the first two hours of life in term and late preterm newborns. The administration of corticosteroids might compensate for the impaired hormonal changes which occur when infants are delivered late preterm, or at term but before the onset of spontaneous labour (elective caesarean section). Corticosteroids might improve the clearance of liquid from the lungs, thus reducing the effort required to breathe and improving respiratory distress.
Objectives: The objective of this review is to assess whether postnatal corticosteroids - compared to placebo, no treatment or any other drugs administered to treat TTN - are effective and safe in the treatment of TTN in infants born at 34 weeks' gestational age or more.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 2), MEDLINE (1996 to 19 February 2019), Embase (1980 to 19 February 2019) and CINAHL (1982 to 19 February 2019). We applied no language restrictions. We searched clinical trial registries for ongoing studies.
Selection criteria: We included randomized controlled trials, quasi-randomized controlled trials and cluster-randomized trials comparing postnatal corticosteroids versus placebo or no treatment or any other drugs administered to infants born at 34 weeks' gestational age or more and less than three days of age with TTN.
Data collection and analysis: For each of the included trials, two review authors independently extracted data (e.g. number of participants, birth weight, gestational age, duration of oxygen therapy, need for continuous positive airway pressure, need for mechanical ventilation, duration of mechanical ventilation, etc.) and assessed the risk of bias (e.g. adequacy of randomization and blinding, completeness of follow-up). The primary outcomes considered in this review were need for nasal continuous positive airway pressure and need for mechanical ventilation. We used the GRADE approach to assess the certainty of the evidence.
Main results: One trial, which included 49 infants, met the inclusion criteria. The trial compared the use of inhaled corticosteroids (budesonide) with placebo. We found no differences between groups in terms of need for nasal continuous positive airway pressure (risk ratio (RR) 1.27, 95% confidence interval (CI) 0.65 to 2.51; 1 study, 49 participants) and need for mechanical ventilation (RR 0.52, 95% CI 0.05 to 5.38; 1 study, 49 participants). The type of mechanical ventilation used in the included study was high-frequency oscillation. Tests for heterogeneity were not applicable for any of the analyses as only one study was included. Out of the secondary outcomes we deemed to be of greatest importance to patients, the study only reported on duration of hospital stay, which was no different between groups. The quality of the evidence is very low, due to the imprecision of the estimates and indirectness. We identified no ongoing trials.
Authors' conclusions: Given the paucity and very low quality of the available evidence, we are unable to determine the benefits and harms of postnatal administration of either inhaled or systemic corticosteroids for the management of TTN.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
MB: no conflict of interest LM: no conflict of interest MGC: no conflict of interest OR: no conflict of interest
Figures
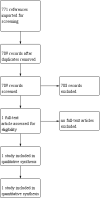
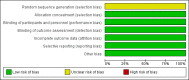



Update of
References
References to studies included in this review
Vaisbourd 2017 {published data only}
Additional references
Avery 1966
-
- Avery ME, Gatewood OB, Brumley G. Transient tachypnea of newborn. Possible delayed resorption of fluid at birth. American Journal of Diseases of Children 1966;111(4):380‐5. [PUBMED: 5906048] - PubMed
Barker 2002
Bayley 1993
-
- Bayley N. Bayley Scales of Infant Development. 2nd Edition. San Antonio (TX): The Psychological Corporation, 1993.
Bayley 2006
-
- Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio (TX): Harcourt Assessment, 2006.
Birnkrant 2006
Clark 2005
Davies 2004
-
- Davies JC. Ion transport in lung disease. Pediatric Pulmonology 2004;26:147‐8. [PUBMED: 15029633] - PubMed
Edwards 2013
Egger 1997
EPOC 2013
-
- Effective Practice, Organisation of Care (EPOC). Data extraction and management. EPOC resources for review authors, 2017. epoc.cochrane.org/epoc‐resources‐review‐authors (accessed 24 May 2017).
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 16 November 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Griffiths 1954
-
- Griffiths R. The Abilities of Babies: A Study in Mental Measurement. New York (NY): McGraw‐Hill Book Co. Inc, 1954.
Guglani 2008
Gupta 2015
Hansen 2008
-
- Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section. BMJ 2008;336(7635):85‐7. [DOI: 10.1136/bmj.39405.539282.BE; PUBMED: 18077440] - DOI - PMC - PubMed
Hibbard 2010
Higgins 2003
Higgins 2017
-
- Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from training.cochrane.org/handbook.
Jacobs 2013
Janer 2015
Jobe 1997
Kassab 2015
Kumar 1996
-
- Kumar A, Bhat BV. Epidemiology of respiratory distress of newborns. Indian Journal of Pediatrics 1996;63(1):93‐8. [PUBMED: 10829971] - PubMed
Liem 2007
Linsell 2016
-
- Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for cerebral palsy and motor impairment in children born very preterm or very low birthweight: a systematic review. Developmental Medicine and Child Neurology 2016;58(6):554‐69. [DOI: 10.1111/dmcn.12972; PUBMED: 26862030] - DOI - PMC - PubMed
Ma 2010
-
- Ma XL, Xu XF, Chen C, Yan CY, Liu YM, Liu L, et al. National Collaborative Study Group for Neonatal Respiratory Distress in Late Preterm or Term Infants. Epidemiology of respiratory distress and the illness severity in late preterm or term infants: a prospective multi‐center study. Chinese Medical Journal 2010;123(20):2776‐80. [PUBMED: 21034581] - PubMed
Mahoney 2013
Miller 1980
-
- Miller LK, Calenoff L, Boehm JJ, Riedy MJ. Respiratory distress in the newborn. JAMA 1980;243(11):1176‐9. [PUBMED: 7359671] - PubMed
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [10.1016/j.jclinepi.2009.06.005; PUBMED: 19631508] - PubMed
Moresco 2016a
Moresco 2016b
Morrison 1995
-
- Morrison JJ, Rennie JM, Milton PJ. Neonatal respiratory morbidity and mode of delivery at term: influence of timing of elective caesarean section. British Journal of Obstetrics and Gynaecology 1995;102(2):101‐6. [PUBMED: 7756199] - PubMed
NICE 2015
-
- National Institute for Health and Care Excellence. Preterm labour and birth: NICE Guideline 25. www.nice.org.uk/guidance/ng25/evidence/full‐guideline‐pdf‐2176838029 (accessed 9 April 2018).
NIH 1994
-
- NIH. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Statement 1994;12(2):1‐24. [PUBMED: 7728157] - PubMed
O'Brodovich 1990
RevMan Web [Computer program]
-
- The Cochrane Collaboration. Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019.
Roberts 2017
Saccone 2016
-
- Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta‐analysis of randomized controlled trials. BMJ (Clinical Research Ed.) 2016;355:i5044. Erratum in: BMJ 2016; 355: i6416 (doi: 10.1136/bmj.i6416). [DOI: 10.1136/bmj.i5044; PUBMED: 27733360] - DOI - PMC - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Sengupta 2013
Silverman 1956
-
- Silverman WA, Andersen DH. A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics 1956;17(1):1‐10. [PUBMED: 13353856] - PubMed
Smith 2000
-
- Smith DE, Otulakowski G, Yeger H, Post M, Cutz E, O'Brodovich HM. Epithelial Na(+) channel (ENaC) expression in the developing normal and abnormal human perinatal lung. American Journal of Respiratory and Critical Care Medicine 2000;161(4 Pt 1):1322‐31. [DOI: 10.1164/ajrccm.161.4.9905064; PUBMED: 10764330] - DOI - PubMed
Sotiriadis 2018
Stark 2001
-
- Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, et al. National Institute of Child Health and Human Development Neonatal Research Network. Adverse effects of early dexamethasone treatment in extremely‐low‐birth‐weight infants. New England Journal of Medicine 2001;344(2):95‐101. [DOI: 10.1056/NEJM200101113440203; PUBMED: 11150359] - DOI - PubMed
Tudehope 1979
-
- Tudehope DI, Smyth MH. Is "transient tachypnoea of the newborn" always a benign disease? Report of 6 babies requiring mechanical ventilation. Australian Paediatric Journal 1979;15(3):160‐5. [PUBMED: 518409] - PubMed
Venkatesh 1997
WHO 2015
-
- World Health Organization. WHO recommendations on interventions to improve preterm birth outcomes. apps.who.int/iris/bitstream/10665/183037/1/9789241508988_eng.pdf (accessed 9 April 2018).
Wood 1972
-
- Wood DW, Downes JJ, Lecks HI. A clinical scoring system for the diagnosis of respiratory failure. Preliminary report on childhood status asthmaticus. American Journal of Diseases of Children 1972;123(3):227‐8. [PUBMED: 5026202] - PubMed
Zubrow 1995
-
- Zubrow AB, Hulman S, Kushner H, Falkner B. Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. Philadelphia Neonatal Blood Pressure Study Group. Journal of Perinatology 1995;15(6):470‐9. [PUBMED: 8648456] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

