Bi-Directional Communication Between Neurons and Astrocytes Modulates Spinal Motor Circuits
- PMID: 32180706
- PMCID: PMC7057799
- DOI: 10.3389/fncel.2020.00030
Bi-Directional Communication Between Neurons and Astrocytes Modulates Spinal Motor Circuits
Abstract
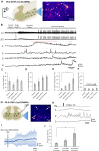
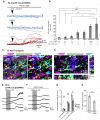
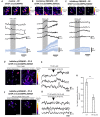
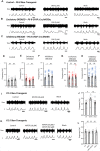
Evidence suggests that astrocytes are not merely supportive cells in the nervous system but may actively participate in the control of neural circuits underlying cognition and behavior. In this study, we examined the role of astrocytes within the motor circuitry of the mammalian spinal cord. Pharmacogenetic manipulation of astrocytic activity in isolated spinal cord preparations obtained from neonatal mice revealed astrocyte-derived, adenosinergic modulation of the frequency of rhythmic output generated by the locomotor central pattern generator (CPG) network. Live Ca2+ imaging demonstrated increased activity in astrocytes during locomotor-related output and in response to the direct stimulation of spinal neurons. Finally, astrocytes were found to respond to neuronally-derived glutamate in a metabotropic glutamate receptor 5 (mGluR5) dependent manner, which in turn drives astrocytic modulation of the locomotor network. Our work identifies bi-directional signaling mechanisms between neurons and astrocytes underlying modulatory feedback control of motor circuits, which may act to constrain network output within optimal ranges for movement.
Keywords: astrocyte; locomotion; mGlu receptor5; neuromodulation; spinal cord.
Copyright © 2020 Broadhead and Miles.
Figures
References
-
- Acevedo J., Santana-Almansa A., Matos-Vergara N., Marrero-Cordero L. R., Cabezas-Bou E., Díaz-Ríos M. (2016). Caffeine stimulates locomotor activity in the mammalian spinal cord via adenosine A1 receptor-dopamine D1 receptor interaction and PKA-dependent mechanisms. Neuropharmacology 101, 490–505. 10.1016/j.neuropharm.2015.10.020 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

