Roles for Countercharge in the Voltage Sensor Domain of Ion Channels
- PMID: 32180723
- PMCID: PMC7059764
- DOI: 10.3389/fphar.2020.00160
Roles for Countercharge in the Voltage Sensor Domain of Ion Channels
Abstract
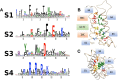
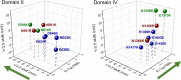
Voltage-gated ion channels share a common structure typified by peripheral, voltage sensor domains. Their S4 segments respond to alteration in membrane potential with translocation coupled to ion permeation through a central pore domain. The mechanisms of gating in these channels have been intensely studied using pioneering methods such as measurement of charge displacement across a membrane, sequencing of genes coding for voltage-gated ion channels, and the development of all-atom molecular dynamics simulations using structural information from prokaryotic and eukaryotic channel proteins. One aspect of this work has been the description of the role of conserved negative countercharges in S1, S2, and S3 transmembrane segments to promote sequential salt-bridge formation with positively charged residues in S4 segments. These interactions facilitate S4 translocation through the lipid bilayer. In this review, we describe functional and computational work investigating the role of these countercharges in S4 translocation, voltage sensor domain hydration, and in diseases resulting from countercharge mutations.
Keywords: channelopathy; countercharge; crystallography; electrostatic; ion channel; molecular dynamics; sliding helix model; voltage sensor domain.
Copyright © 2020 Groome and Bayless-Edwards.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

