Direct-acting Anticoagulants in Chronic Coronary Syndromes
- PMID: 32180831
- PMCID: PMC7066807
- DOI: 10.15420/ecr.2018.24.2
Direct-acting Anticoagulants in Chronic Coronary Syndromes
Abstract
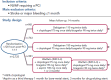
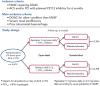
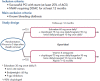
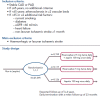
Direct-acting oral anticoagulants (DOACs) are easier to use, safer than and as effective as vitamin K antagonists (VKA) in the treatment of non-valvular AF (NVAF). Because of their favourable safety profile and easier use than VKAs, DOACs as anti-thrombotic therapy may have a role in the management of chronic coronary syndromes (CCS). To date, few studies have evaluated DOACs in this setting. Initial studies have focused on patients receiving DOACs for NVAF undergoing acute or elective percutaneous coronary intervention who additionally require dual antiplatelet therapy (DAPT). Rivaroxaban 15 mg once daily plus a P2Y12 inhibitor compared with a VKA regimen was associated with a reduction of bleedings (HR 0.59; 95% CI [0.47-0.76]; p<0.001). Rivaroxaban 2.5 mg twice daily plus DAPT up to 12 months followed by rivaroxaban 15 mg once daily plus P2Y12 inhibitor showed similar results. Dabigatran 110 mg twice daily plus a P2Y12 inhibitor versus a VKA regimen was associated with a reduction of bleedings (HR 0.52; 95% CI [0.42-0.63]; p<0.001), after a mean follow-up of 14 months. A dabigatran 150 mg regimen showed similar results. Apixaban 5 mg twice daily plus a P2Y12 inhibitor versus a VKA regimen confirmed at 6 months the safety of DOACs with a reduction of bleedings (HR 0.69; 95% CI [0.58-0.81]; p<0.001 for non-inferiority and superiority). Edoxaban 60 mg once daily plus a P2Y12 inhibitor was non-inferior to a VKA regimen on bleeding outcomes (major bleeding or non-major clinically relevant non-major bleeding) after a 12-month follow-up (HR 0.83; 95% CI [0.65-1.05]; p=0.001 for non-inferiority; p=0.1154 for superiority). Meta-analysis of these four trials confirmed the safety of DOACs regarding bleeding outcomes, but showed a trend toward stent thrombosis for dual antithrombotic therapy using DOACs versus triple antithrombotic therapy using VKAs. DOACs may show promise in the management of high-risk patients with chronic coronary syndromes. In these patients, rivaroxaban 2.5 mg twice daily in addition to aspirin was shown to reduce the composite outcome of cardiovascular death, stroke or MI compared to aspirin alone (HR 0.76; 95% CI [0.66-0.86]; p<0.001). All-cause death, cardiovascular death and stroke were also significantly lower. This benefit was at the cost of an increase in non-fatal bleeding.
Keywords: Chronic coronary syndromes; direct oral anticoagulants; non-valvular AF; percutaneous coronary intervention; secondary prevention.
Copyright © 2020, Radcliffe Cardiology.
Conflict of interest statement
Disclosure: ES has received fees for lecture and consulting from AstraZeneca, Bayer, BMS, MSD, Novartis, Roche Diagnostics and Servier. PGS discloses research grants from Bayer, Merck, Sanofi, and Servier and speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Boehringer-Ingelheim, Bristol-Myers-Squibb, Lilly, Merck, Novartis, Novo-Nordisk, Pfizer, Regeneron, Sanofi and Servier.
Figures



References
-
- Naghavi M, Wang H, Lozano R et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. - DOI - PMC - PubMed
-
- Montalescot G, Sechtem U, Achenbach S et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

