Recent developments in photoredox-catalyzed remote ortho and para C-H bond functionalizations
- PMID: 32180843
- PMCID: PMC7059497
- DOI: 10.3762/bjoc.16.26
Recent developments in photoredox-catalyzed remote ortho and para C-H bond functionalizations
Abstract
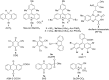
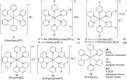
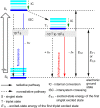
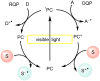
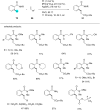
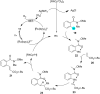
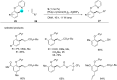
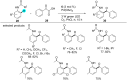
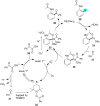
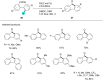
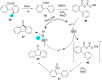
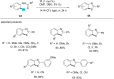
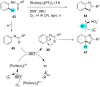
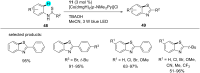
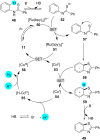
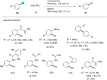
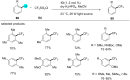
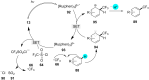
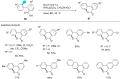
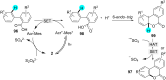
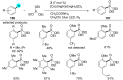
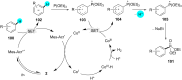
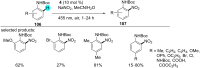
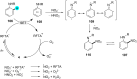
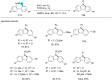
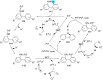
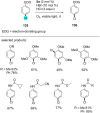
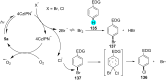
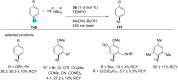
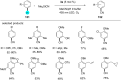
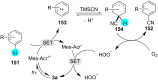
In recent years, the research area of direct C-H bond functionalizations was growing exponentially not only due to the ubiquity of inert C-H bonds in diverse organic compounds, including bioactive natural and nonnatural products, but also due to its impact on the discovery of pharmaceutical candidates and the total synthesis of intricate natural products. On the other hand, more recently, the field of photoredox catalysis has become an indispensable and unparalleled research topic in modern synthetic organic chemistry for the constructions of challenging bonds, having the foremost scope in academia, pharmacy, and industry. Therefore, the development of green, simpler, and effective methodologies to accomplish direct C-H bond functionalization is well overdue and highly desirable to the scientific community. In this review, we mainly highlight the impact on, and the utility of, photoredox catalysts in inert ortho and para C-H bond functionalizations. Although a surge of research papers, including reviews, demonstrating C-H functionalizations have been published in this vital area of research, to our best knowledge, this is the first review that focuses on ortho and para C-H functionalizations by photoredox catalysis to provide atom- and step-economic organic transformations. We are certain that this review will act as a promoter to highlight the application of photoredox catalysts for the functionalization of inert bonds in the domain of synthetic organic chemistry.
Keywords: dual catalysis; light; ortho and para C–H bond functionalization; photoredox catalysis.
Copyright © 2020, Siddiqui and Ali; licensee Beilstein-Institut.
Figures
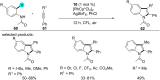
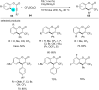
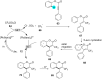
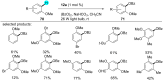
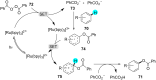
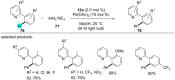
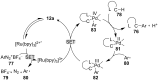
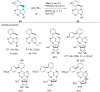
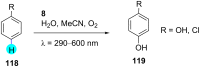
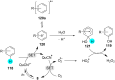
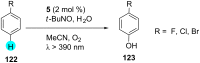
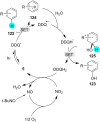
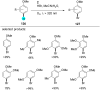
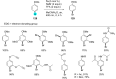
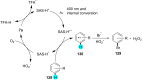
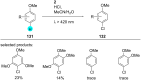
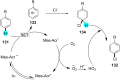
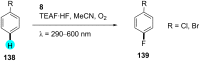
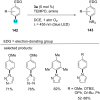
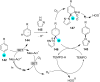
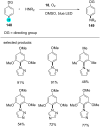
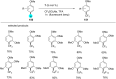
References
Publication types
LinkOut - more resources
Full Text Sources
