Aqueous zinc ion batteries: focus on zinc metal anodes
- PMID: 32180925
- PMCID: PMC7053421
- DOI: 10.1039/d0sc00022a
Aqueous zinc ion batteries: focus on zinc metal anodes
Abstract
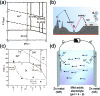
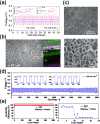
Despite the prevalence of lithium ion batteries in modern technology, the search for alternative electrochemical systems to complement the global battery portfolio is an ongoing effort. The search has resulted in numerous candidates, among which mildly acidic aqueous zinc ion batteries have recently garnered significant academic interest, mostly due to their inherent safety. As the anode is often fixed as zinc metal in these systems, most studies address the absence of a suitable cathode for reaction with zinc ions. This has led to aggressive research into viable intercalation cathodes, some of which have shown impressive results. However, many investigations often overlook the implications of the zinc metal anode, when in fact the anode is key to determining the energy density of the entire cell. In this regard, we aim to shed light on the importance of the zinc metal anode. This perspective offers a brief discussion of zinc electrochemistry in mildly acidic aqueous environments, along with an overview of recent efforts to improve the performance of zinc metal to extract key lessons for future research initiatives. Furthermore, we discuss the energy density ramifications of the zinc anode with respect to its weight and reversibility through simple calculations for numerous influential reports in the field. Finally, we offer some perspectives on the importance of optimizing zinc anodes as well as a future direction for developing high-performance aqueous zinc ion batteries.
This journal is © The Royal Society of Chemistry 2020.
Figures





References
-
- Wang J., Yamada Y., Sodeyama K., Watanabe E., Takada K., Tateyama Y., Yamada A. Nat. Energy. 2018;3:22–29.
-
- Kim H., Hong J., Park K.-Y., Kim H., Kim S.-W., Kang K. Chem. Rev. 2014;114:11788–11827. - PubMed
-
- Konarov A., Voronina N., Jo J. H., Bakenov Z., Sun Y.-K., Myung S.-T. ACS Energy Lett. 2018;3:2620–2640.
-
- Song M., Tan H., Chao D., Fan H. J. Adv. Funct. Mater. 2018;28:1802564.
-
- Zhang X. G., Corrosion and Electrochemistry of Zinc, Springer, 1996.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

