An update on the genetics and pathogenesis of hereditary angioedema
- PMID: 32181278
- PMCID: PMC7063419
- DOI: 10.1016/j.gendis.2019.07.002
An update on the genetics and pathogenesis of hereditary angioedema
Abstract
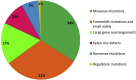
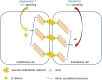
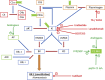
Hereditary angioedema (HAE) is an uncommon genetic disorder characterized by recurrent episodes of edema involving subcutaneous tissue and submucosa. The pathogenesis of HAE reflects an intricate coordinated regulation of components of complement, kinin and hemostatic pathway. Till date, mutations in 4 different genes have been identified to cause HAE which includes serine protease inhibitor G1 (SERPING1), factor XII (F12), plasminogen (PLG) and angiopoietin 1 (ANGPT 1). These mutations lead to increased bradykinin 2 receptor mediated signalling via increased production of bradykinin except mutations in ANGPT1 gene that disturbs the cytoskeletal assembly of vascular endothelial cells. In this review we aim to summarize the recent advances in the pathogenesis and genetics of HAE. We also provide an overview of possible future prospects in the identification of new genetic defects in HAE.
Keywords: Angiopoietin 1; C1 inhibitor; Factor XII; Genetics; Hereditary angioedema; Plasminogen.
© 2019 Chongqing Medical University. Production and hosting by Elsevier B.V.
Figures

References
-
- Jalaj S., Scolapio J.S. Gastrointestinal manifestations, diagnosis, and management of hereditary angioedema. J Clin Gastroenterol. 2013;47:817–823. - PubMed
-
- Xu Y.Y., Zhi Y.X., Liu R.L. Upper airway edema in 43 patients with hereditary angioedema. Ann Allergy Asthma Immunol. 2014;112(6):539–544. - PubMed
-
- Zanichelli A., Longhurst H.J., Maurer M. Misdiagnosis trends in patients with hereditary angioedema from the real-world clinical setting. Ann Allergy Asthma Immunol. 2016;117(4):394–398. - PubMed
-
- Zuraw B.L., Christiansen S.C. HAE pathophysiology and underlying mechanisms. Clin Rev Allergy Immunol. 2016;51(2):216–229. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

