In silico experiments of bone remodeling explore metabolic diseases and their drug treatment
- PMID: 32181336
- PMCID: PMC7060067
- DOI: 10.1126/sciadv.aax0938
In silico experiments of bone remodeling explore metabolic diseases and their drug treatment
Abstract
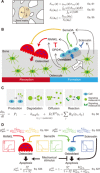
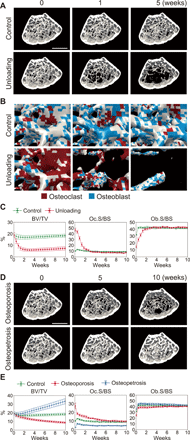
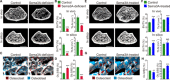
Bone structure and function are maintained by well-regulated bone metabolism and remodeling. Although the underlying molecular and cellular mechanisms are now being understood, physiological and pathological states of bone are still difficult to predict due to the complexity of intercellular signaling. We have now developed a novel in silico experimental platform, V-Bone, to integratively explore bone remodeling by linking complex microscopic molecular/cellular interactions to macroscopic tissue/organ adaptations. Mechano-biochemical couplings modeled in V-Bone relate bone adaptation to mechanical loading and reproduce metabolic bone diseases such as osteoporosis and osteopetrosis. V-Bone also enables in silico perturbation on a specific signaling molecule to observe bone metabolic dynamics over time. We also demonstrate that this platform provides a powerful way to predict in silico therapeutic effects of drugs against metabolic bone diseases. We anticipate that these in silico experiments will substantially accelerate research into bone metabolism and remodeling.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


Comment in
-
In silico tools predict effects of drugs on bone remodelling.Nat Rev Rheumatol. 2020 Sep;16(9):475-476. doi: 10.1038/s41584-020-0457-6. Nat Rev Rheumatol. 2020. PMID: 32581392 No abstract available.
References
-
- Cowin S. C., How is a tissue built? J. Biomech. Eng. 122, 553–569 (2000). - PubMed
-
- van der Meulen M. C. H., Huiskes R., Why mechanobiology? A survey article. J. Biomech. 35, 401–414 (2002). - PubMed
-
- Seeman E., Delmas P. D., Bone quality—The material and structural basis of bone strength and fragility. New Engl. J. Med. 354, 2250–2261 (2006). - PubMed
-
- Teitelbaum S. L., Ross F. P., Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 4, 638–649 (2003). - PubMed
-
- Boyle W. J., Simonet W. S., Lacey D. L., Osteoclast differentiation and activation. Nature 423, 337–342 (2003). - PubMed

