Probing biophysical sequence constraints within the transmembrane domains of rhodopsin by deep mutational scanning
- PMID: 32181350
- PMCID: PMC7056298
- DOI: 10.1126/sciadv.aay7505
Probing biophysical sequence constraints within the transmembrane domains of rhodopsin by deep mutational scanning
Abstract
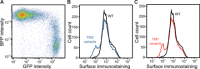
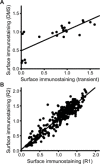
Membrane proteins must balance the sequence constraints associated with folding and function against the hydrophobicity required for solvation within the bilayer. We recently found the expression and maturation of rhodopsin are limited by the hydrophobicity of its seventh transmembrane domain (TM7), which contains polar residues that are essential for function. On the basis of these observations, we hypothesized that rhodopsin's expression should be less tolerant of mutations in TM7 relative to those within hydrophobic TM domains. To test this hypothesis, we used deep mutational scanning to compare the effects of 808 missense mutations on the plasma membrane expression of rhodopsin in HEK293T cells. Our results confirm that a higher proportion of mutations within TM7 (37%) decrease rhodopsin's plasma membrane expression relative to those within a hydrophobic TM domain (TM2, 25%). These results in conjunction with an evolutionary analysis suggest solvation energetics likely restricts the evolutionary sequence space of polar TM domains.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution License 4.0 (CC BY).
Figures




References
-
- Bershtein S., Segal M., Bekerman R., Tokuriki N., Tawfik D. S., Robustness-epistasis link shapes the fitness landscape of a randomly drifting protein. Nature 444, 929–932 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

