Complement in malaria: immune evasion strategies and role in protective immunity
- PMID: 32181490
- PMCID: PMC8653895
- DOI: 10.1002/1873-3468.13772
Complement in malaria: immune evasion strategies and role in protective immunity
Abstract
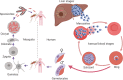
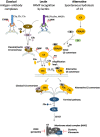
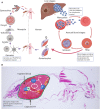
The malaria parasite has for long been thought to escape host complement attack as a survival strategy. However, it was only recently that complement evasion mechanisms of the parasite were described. Simultaneously, the role of complement in antibody-mediated naturally acquired and vaccine-induced protection against malaria has also been reported. Such findings should be considered in future vaccine design, given the current need to develop more efficacious vaccines against malaria. Parasite antigens derived from molecules mediating functions crucial for parasite survival, such as complement evasion, or parasite antigens against which antibody responses lead to an efficient complement attack could present new candidates for vaccines. In this review, we discuss recent findings on complement evasion by the malaria parasites and the emerging role of complement in antibody-mediated protection against malaria. We emphasize that immune responses to vaccines based on complement inhibitors should not only induce complement-activating antibodies but also neutralize the escape mechanisms of the parasite.
Keywords: Plasmodium; complement; immunity; malaria.
© 2020 The Authors. FEBS Letters published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures

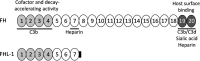

References
-
- WHO (2019) WHO | World malaria report 2019. WHO.
-
- White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM and White NJ (2014) Malaria. Lancet 383, 723–735. - PubMed
-
- Rosenberg R, Burge R and Schneider I (1990) An estimation of the number of malaria sporozoites ejected by a feeding mosquito. Trans R Soc Trop Med Hyg 84, 209–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

