The "Green" Electrochemical Synthesis of Periodate
- PMID: 32181555
- PMCID: PMC7317427
- DOI: 10.1002/anie.202002717
The "Green" Electrochemical Synthesis of Periodate
Abstract
High-grade periodate is relatively expensive, but is required for many sensitive applications such as the synthesis of active pharmaceutical ingredients. These high costs originate from using lead dioxide anodes in contemporary electrochemical methods and from expensive starting materials. A direct and cost-efficient electrochemical synthesis of periodate from iodide, which is less costly and relies on a readily available starting material, is reported. The oxidation is conducted at boron-doped diamond anodes, which are durable, metal-free, and nontoxic. The avoidance of lead dioxide ultimately lowers the cost of purification and quality assurance. The electrolytic process was optimized by statistical methods and was scaled up in an electrolysis flow cell that enhanced the space-time yields by a cyclization protocol. An LC-PDA analytical protocol was established enabling simple quantification of iodide, iodate, and periodate simultaneously with remarkable precision.
Keywords: boron-doped diamond; electrolysis; flow chemistry; oxidation; periodate.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

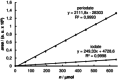

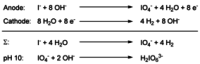
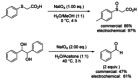
References
-
- None
-
- Sudalai A., Khenkin A., Neumann R., Org. Biomol. Chem. 2015, 13, 4374–4394; - PubMed
-
- Fuchs P. L., Charette A. B., Rovis T., Bode J. W., Essential Reagents for Organic Synthesis, Wiley-VCH, Weinheim, 2016.
-
- None
-
- Abdel-Akher M., Smith F., J. Am. Chem. Soc. 1959, 81, 1718–1721;
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

