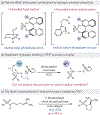
Enantioselective Hydroamination of Alkenes with Sulfonamides Enabled by Proton-Coupled Electron Transfer
- PMID: 32182054
- PMCID: PMC7468321
- DOI: 10.1021/jacs.0c01332
Enantioselective Hydroamination of Alkenes with Sulfonamides Enabled by Proton-Coupled Electron Transfer
Abstract
An enantioselective, radical-based method for the intramolecular hydroamination of alkenes with sulfonamides is reported. These reactions are proposed to proceed via N-centered radicals formed by proton-coupled electron transfer (PCET) activation of sulfonamide N-H bonds. Noncovalent interactions between the neutral sulfonamidyl radical and a chiral phosphoric acid generated in the PCET event are hypothesized to serve as the basis for asymmetric induction in a subsequent C-N bond forming step, achieving selectivities of up to 98:2 er. These results offer further support for the ability of noncovalent interactions to enforce stereoselectivity in reactions of transient and highly reactive open-shell intermediates.
Figures
References
-
- Knowles RR; Jacobsen EN Attractive Noncovalent Interactions in Asymmetric Catalysis: Links Between Enzymes and Small Molecule Catalysts. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 20678–20685. - PMC - PubMed
- Davis HJ; Phipps RJ Harnessing non-covalent interactions to exert control over regioselectivity and site-selectivity in catalytic reactions. Chem. Sci 2017, 8, 864–877. - PMC - PubMed
- Raynal M; Ballester P; Vidal-Ferran A; van Leeuwen PWNM Supramolecular catalysis. Part 2: artificial enzyme mimics. Chem. Soc. Rev 2014, 43, 1734–1787. - PubMed
- Taylor MS; Jacobsen EN Asymmetric Catalysis by Chiral Hydrogen-Bond Donors. Angew, Chem. Int. Ed 2006, 45, 1520–1543. - PubMed
-
- For studies of hydrogen bonding to radicals, see: Johnson ER; Salamone M; Bietti M; DiLabio GA Modeling Noncovalent Radical-Molecule Interactions Using Convetional Density-Functional Theory: Beware Erroneous Charge Transfer. J. Phys Chem. A 2014, 117, 947–952. - PubMed
- Hernández-Soto H; Weinhold F; Francisco JS Radical hydrogen bonding: Origins of stability of radical-molecule complexes. J. Chem. Phys 2007, 127, 164102. - PubMed
- Johnson ER; DiLabio GA Radicals as Hydrogen Bond Donors and Acceptors. Interdiscip. Sci. Comput. Life Sci 2009, 1, 133–140. - PubMed
-
- For general reviews of enantioselective radical reactions. Studer A; Curran DP Catalysis of Radical Reactions: A Radical Chemistry Perspective. Angew. Chem. Int. Ed 2016, 55, 58–102. - PubMed
- Sibi MP; Manyem S; Zimmerman J Enantioselective Radical Processes. Chem. Rev 2003, 103, 3263–3295. - PubMed
- Zhang L; Meggers E Steering Asymmetric Lewis Acid Catalysis Exclusively with Octahedral Metal-Centered Chirality. Acc. Chem. Res 2017, 50, 320–330. - PubMed
- Yoon TP Photochemical Stereocontrol Using Tandem Photoredox-Chiral Lewis Acid Catalysis. Acc. Chem. Res 2016, 49, 2307–2315. - PMC - PubMed
- Silvi M; Melchiorre P Enhancing the potential of enantioselective organocatalysis with light. Nature, 2018, 554, 41–49. - PubMed
-
- For examples of a role for hydrogen bonding in radical reactions: Waldner A; De Mesmaeker A; Hoffmann P; Mindt T; Winkler T α-Sulfinyl Substituted Radicals; 1. Stereoselective Radical Addition Reactions of Cyclic α-Sulfinyl Radicals. Synlett, 1991, 2, 101–104.
- De Mesmaeker A; Waldner A; Hoffmann P; Mindt T α-Sulfinyl Substituted Radicals; II. Stereoselective Inter- and Intramolecular Addition Reactions of Acyclic α-Sulfinyl Radicals. Synlett, 1993, 11, 871–874.
- Curran DP; Kuo LH Altering the stereochemistry of Allylation Reactions of Cyclic α-Sulfinyl Radicals with Diarylureas. J. Org. Chem 1994, 59, 3259–3261.
- Böhm A; Bach T Synthesis of Supramolecular Iridium Catalysts and Their Use in Enantioselective Visible-Light-Induced Reactions. Synlett, 2016, 27, 1056–1060.
- Bauer A; Westkämper F; Grimme S; Bach T Catalytic enantioselective Reactions Driven by Photoinduced Electron Transfer. Nature 2005, 436, 1139–1140. - PubMed
- Bach T; Bergmann H; Grosch B; Harms K Highly Enantioselective Intra- and Intermolecular [2 + 2] Photocycloaddition Reactions of 2-Quinolones Mediated by a Chiral Lactam Host: Host−Guest Interactions, Product Configuration, and the Origin of the Stereoselectivity in Solution. J. Am. Chem. Soc 2002, 124, 7982–7990. - PubMed
- Aechtner T; Dressel M; Bach T Hydrogen Bond Mediated Enantioselectivity of Radical Reactions. Angew. Chem. Int. Ed 2004, 43, 5849–5851. - PubMed
- Müller C; Bauer A; Bach T Light-Driven Enantioselective Organocatalysis. Angew. Chem., Int. Ed 2009, 48, 6640–6642. - PubMed
- Sandoval BA; Meichan AJ; Hyster TK Enantioselective Hydrogen Atom Transfer: Discovery of Catalytic Promiscuity in Flavin-Dependent ‘Ene’-Reductases. J. Am. Chem. Soc 2017, 139, 11313–11316. - PubMed
- Proctor RSJ; Davis HJ Phipps RJ Catalytic enantioselective Minisci-Type addition to heteroarenes. Science, 2018, 360, 419–422. - PubMed
- Uraguchi D; Kinoshita N; Kizu T; Ooi T Synergistic Catalysis of Ionic Brønsted Acid and Photosensitizer for a Redox Neutral Asymmetric α-Coupling of N-Arylaminomethanes with Aldimines. J. Am. Chem. Soc 2015, 137, 13768–13771. - PubMed
- Shevchenko GA; Oppelaar B; List B An Unexpected α-Oxidation of Cyclic Ketones with 1,4-Benzoquinone by Enol Catalysis. Angew. Chem. Int. Ed 2018, 57, 10756–10759. - PubMed
- Cao K; Tan SM; Lee R; Yang S; Jia H; Zhao X; Qiao B; Jiang Z Catalytic Enantioselective Addition of Prochiral Racicals to Vinylpyridines. J. Am. Chem. Soc 2019, 141, 5437–5443. - PubMed
-
- Weinberg DR; Gagliardi CJ; Hull JF; Murphy CF; Kent CA; Westlake BC; Paul A; Ess DH; McCafferty DG; Meyer TJ Proton-Coupled Electron Transfer. Chem. Rev 2012, 112, 4016–4093. - PubMed
- Mayer JM Proton-Coupled Electron Transfer: A Reaction Chemist’s View. Annu. Rev. Phys. Chem 2004, 55, 363–390. - PubMed
- Hoffmann N Proton-Coupled Electron Transfer in Photoredox Catalytic Reactions. Eur. J. Org. Chem 2017, 15, 1982–1992.
- Miller DC; Tarantino KT; Knowles RR Proton-Coupled Electron Transfer in Organic Synthesis: Fundamentals, Applications, and Opportunities. Top. Curr. Chem 2016, 374, 30. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources