The Transient Receptor Potential Channel Vanilloid 1 Is Critical in Innate Airway Epithelial Responses to Protease Allergens
- PMID: 32182090
- PMCID: PMC7397771
- DOI: 10.1165/rcmb.2019-0170OC
The Transient Receptor Potential Channel Vanilloid 1 Is Critical in Innate Airway Epithelial Responses to Protease Allergens
Abstract
The airway epithelium plays a critical role in innate responses to airborne allergens by secreting IL-1 family cytokines such as IL-1α and IL-33 as alarmins that subsequently orchestrate appropriate immune responses. Previous studies revealed that epithelial IL-33 secretion by allergens such as Alternaria alternata or house dust mite involves Ca2+-dependent signaling, via initial activation of ATP-stimulated P2YR2 (type 2 purinoceptor) and subsequent activation of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase DUOX1. We sought to identify proximal mechanisms by which epithelial cells sense these allergens and here highlight the importance of PAR2 (protease-activated receptor 2) and TRP (transient receptor potential) Ca2+ channels such as TRPV1 (TRP vanilloid 1) in these responses. Combined studies of primary human nasal and mouse tracheal epithelial cells, as well as immortalized human bronchial epithelial cells, indicated the importance of both PAR2 and TRPV1 in IL-33 secretion by both Alternaria alternata and house dust mite, based on both pharmacological and genetic approaches. TRPV1 was also critically involved in allergen-induced ATP release, activation of DUOX1, and redox-dependent activation of EGFR (epidermal growth factor receptor). Moreover, genetic deletion of TRPV1 dramatically attenuated allergen-induced IL-33 secretion and subsequent type 2 responses in mice in vivo. TRPV1 not only contributed to ATP release and P2YR2 signaling but also was critical in downstream innate responses to ATP, indicating potentiating effects of P2YR2 on TRPV1 activation. In aggregate, our studies illustrate a complex relationship between various receptor types, including PAR2 and P2YR2, in epithelial responses to asthma-relevant airborne allergens and highlight the central importance of TRPV1 in such responses.
Keywords: ATP; DUOX1; IL-33; lung; type 2 immune responses.
Figures


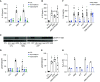



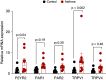
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

