The potential role of using vaccine patches to induce immunity: platform and pathways to innovation and commercialization
- PMID: 32182145
- PMCID: PMC7814398
- DOI: 10.1080/14760584.2020.1732215
The potential role of using vaccine patches to induce immunity: platform and pathways to innovation and commercialization
Abstract
Introduction: In the last two decades, the evidence related to using vaccine patches with multiple short projections (≤1 mm) to deliver vaccines through the skin increased significantly and demonstrated their potential as an innovative delivery platform.Areas covered: We review the vaccine patch literature published in English as of 1 March 2019, as well as available information from key stakeholders related to vaccine patches as a platform. We identify key research topics related to basic and translational science on skin physical properties and immunobiology, patch development, and vaccine manufacturing.Expert opinion: Currently, vaccine patch developers continue to address some basic science and other platform issues in the context of developing a potential vaccine patch presentation for an existing or new vaccine. Additional clinical data and manufacturing experience could shift the balance toward incentivizing existing vaccine manufactures to further explore the use of vaccine patches to deliver their products. Incentives for innovation of vaccine patches differ for developed and developing countries, which will necessitate different strategies (e.g. public-private partnerships, push, or pull mechanisms) to support the basic and applied research needed to ensure a strong evidence base and to overcome translational barriers for vaccine patches as a delivery platform.
Keywords: Vaccine; human skin; microarray patch; microneedle.
Conflict of interest statement
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures

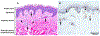

Similar articles
-
Microneedle Patches as Drug and Vaccine Delivery Platform.Curr Med Chem. 2017;24(22):2413-2422. doi: 10.2174/0929867324666170526124053. Curr Med Chem. 2017. PMID: 28552053 Review.
-
Engineering Microneedle Patches for Vaccination and Drug Delivery to Skin.Annu Rev Chem Biomol Eng. 2017 Jun 7;8:177-200. doi: 10.1146/annurev-chembioeng-060816-101514. Epub 2017 Mar 24. Annu Rev Chem Biomol Eng. 2017. PMID: 28375775 Review.
-
Dissolving Microneedle Patches for Dermal Vaccination.Pharm Res. 2017 Nov;34(11):2223-2240. doi: 10.1007/s11095-017-2223-2. Epub 2017 Jul 17. Pharm Res. 2017. PMID: 28718050 Free PMC article. Review.
-
Potential use of microarray patches for vaccine delivery in low- and middle- income countries.Vaccine. 2019 Jul 26;37(32):4427-4434. doi: 10.1016/j.vaccine.2019.03.035. Epub 2019 Jun 28. Vaccine. 2019. PMID: 31262587 Review.
-
Rabies vaccination in dogs using a dissolving microneedle patch.J Control Release. 2016 Oct 10;239:19-26. doi: 10.1016/j.jconrel.2016.08.012. Epub 2016 Aug 11. J Control Release. 2016. PMID: 27524283
Cited by
-
Technological Approaches for Improving Vaccination Compliance and Coverage.Vaccines (Basel). 2020 Jun 16;8(2):304. doi: 10.3390/vaccines8020304. Vaccines (Basel). 2020. PMID: 32560088 Free PMC article. Review.
-
Evaluation of the Delivery of a Live Attenuated Porcine Reproductive and Respiratory Syndrome Virus as a Unit Solid Dose Injectable Vaccine.Vaccines (Basel). 2022 Oct 30;10(11):1836. doi: 10.3390/vaccines10111836. Vaccines (Basel). 2022. PMID: 36366345 Free PMC article.
-
A Health Economic Analysis for Oral Poliovirus Vaccine to Prevent COVID-19 in the United States.Risk Anal. 2021 Feb;41(2):376-386. doi: 10.1111/risa.13614. Epub 2020 Oct 20. Risk Anal. 2021. PMID: 33084153 Free PMC article. Review.
-
Immunologic mechanisms of seasonal influenza vaccination administered by microneedle patch from a randomized phase I trial.NPJ Vaccines. 2021 Jul 14;6(1):89. doi: 10.1038/s41541-021-00353-0. NPJ Vaccines. 2021. PMID: 34262052 Free PMC article.
-
Transdermal approaches to vaccinations in the COVID-19 pandemic era.Ther Adv Vaccines Immunother. 2021 Aug 21;9:25151355211039073. doi: 10.1177/25151355211039073. eCollection 2021. Ther Adv Vaccines Immunother. 2021. PMID: 34447901 Free PMC article. Review.
References
-
- Papania M, Zehrung D, Jarrahian C. Technologies to Improve Immunization In: Plotkin’s Vaccines. Plotkin S, Orenstein W, Offit P, Edwards K (Ed.^(Eds) (Elsevier, Philadelphia, PA, 2017) 1320–1353.
-
- Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine, 28(51), 8049–8052 (2010). - PubMed
-
- Tuft L, Yagle EM, Rogers S. Comparative Study of the Antibody Response After Various Methods of Administration of Mixed Typhoid Vaccine: With Particular Reference to the Intradermal and Oral Methods*. The Journal of Infectious Diseases, 50(2), 98–110 (1932).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
