Neutralizing antibody VRC01 failed to select for HIV-1 mutations upon viral rebound
- PMID: 32182219
- PMCID: PMC7259993
- DOI: 10.1172/JCI134395
Neutralizing antibody VRC01 failed to select for HIV-1 mutations upon viral rebound
Abstract
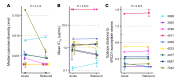
Infusion of the broadly neutralizing antibody VRC01 has been evaluated in individuals chronically infected with HIV-1. Here, we studied how VRC01 infusions affected viral rebound after cessation of antiretroviral therapy (ART) in 18 acutely treated and durably suppressed individuals. Viral rebound occurred in all individuals, yet VRC01 infusions modestly delayed rebound and participants who showed a faster decay of VRC01 in serum rebounded more rapidly. Participants with strains most sensitive to VRC01 or with VRC01 epitope motifs similar to known VRC01-susceptible strains rebounded later. Upon rebound, HIV-1 sequences were indistinguishable from those sampled at diagnosis. Across the cohort, participant-derived Env showed different sensitivity to VRC01 neutralization (including 2 resistant viruses), yet neutralization sensitivity was similar at diagnosis and after rebound, indicating the lack of selection for VRC01 resistance during treatment interruption. Our results showed that viremia rebounded despite the absence of HIV-1 adaptation to VRC01 and an average VRC01 trough of 221 μg/mL. Although VRC01 levels were insufficient to prevent a resurgent infection, knowledge that they did not mediate Env mutations in acute-like viruses is relevant for antibody-based strategies in acute infection.
Keywords: AIDS/HIV; Adaptive immunity; Bioinformatics.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

