Leptin receptor-expressing nucleus tractus solitarius neurons suppress food intake independently of GLP1 in mice
- PMID: 32182221
- PMCID: PMC7205255
- DOI: 10.1172/jci.insight.134359
Leptin receptor-expressing nucleus tractus solitarius neurons suppress food intake independently of GLP1 in mice
Abstract
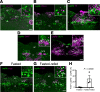
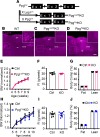
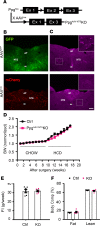
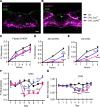
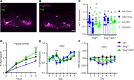
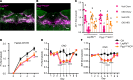
Leptin receptor-expressing (LepRb-expressing) neurons of the nucleus tractus solitarius (NTS; LepRbNTS neurons) receive gut signals that synergize with leptin action to suppress food intake. NTS neurons that express preproglucagon (Ppg) (and that produce the food intake-suppressing PPG cleavage product glucagon-like peptide-1 [GLP1]) represent a subpopulation of mouse LepRbNTS cells. Using Leprcre, Ppgcre, and Ppgfl mouse lines, along with Designer Receptors Exclusively Activated by Designer Drugs (DREADDs), we examined roles for Ppg in GLP1NTS and LepRbNTS cells for the control of food intake and energy balance. We found that the cre-dependent ablation of NTS Ppgfl early in development or in adult mice failed to alter energy balance, suggesting the importance of pathways independent of NTS GLP1 for the long-term control of food intake. Consistently, while activating GLP1NTS cells decreased food intake, LepRbNTS cells elicited larger and more durable effects. Furthermore, while the ablation of NTS Ppgfl blunted the ability of GLP1NTS neurons to suppress food intake during activation, it did not impact the suppression of food intake by LepRbNTS cells. While Ppg/GLP1-mediated neurotransmission plays a central role in the modest appetite-suppressing effects of GLP1NTS cells, additional pathways engaged by LepRbNTS cells dominate for the suppression of food intake.
Keywords: Endocrinology; Leptin; Metabolism; Obesity; Peptides.
Conflict of interest statement
Figures
References
-
- Grill HJ, Hayes MR. The nucleus tractus solitarius: a portal for visceral afferent signal processing, energy status assessment and integration of their combined effects on food intake. Int J Obes (Lond) 2009;33(Suppl_1):S11–S15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

