Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation
- PMID: 32182223
- PMCID: PMC7324171
- DOI: 10.1172/JCI129401
Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation
Abstract
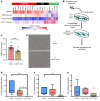
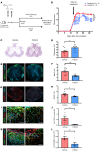
Multiple sclerosis (MS) is an inflammatory demyelinating disorder of the CNS. Bile acids are cholesterol metabolites that can signal through receptors on cells throughout the body, including in the CNS and the immune system. Whether bile acid metabolism is abnormal in MS is unknown. Using global and targeted metabolomic profiling, we identified lower levels of circulating bile acid metabolites in multiple cohorts of adult and pediatric patients with MS compared with controls. In white matter lesions from MS brain tissue, we noted the presence of bile acid receptors on immune and glial cells. To mechanistically examine the implications of lower levels of bile acids in MS, we studied the in vitro effects of an endogenous bile acid, tauroursodeoxycholic acid (TUDCA), on astrocyte and microglial polarization. TUDCA prevented neurotoxic (A1) polarization of astrocytes and proinflammatory polarization of microglia in a dose-dependent manner. TUDCA supplementation in experimental autoimmune encephalomyelitis reduced the severity of disease through its effects on G protein-coupled bile acid receptor 1 (GPBAR1). We demonstrate that bile acid metabolism was altered in MS and that bile acid supplementation prevented polarization of astrocytes and microglia to neurotoxic phenotypes and ameliorated neuropathology in an animal model of MS. These findings identify dysregulated bile acid metabolism as a potential therapeutic target in MS.
Keywords: Metabolism; Multiple sclerosis; Neurodegeneration; Neuroscience.
Conflict of interest statement
Figures